Cetilistat medicinal composition and preparation method thereof
A technology of new lisstat and its composition, which is applied in the field of drug combination and preparation of new lisstat tablets, can solve the problems such as easy adhesion of the main drug, achieve good dissolution effect, simple preparation process, and increase the effect of impurities
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
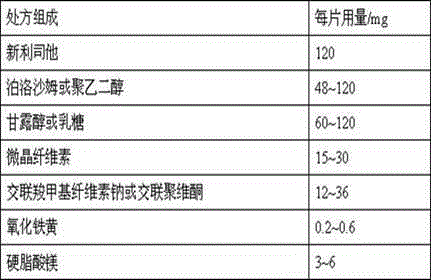
[0022] 1) Prescription composition
[0023] prescription composition 500 tablets / g Neolistat 60 polyethylene glycol 6000 24 Mannitol 36 microcrystalline cellulose 7.5 Croscarmellose Sodium 11 Iron oxide yellow 0.2
[0025] 2) Preparation method
[0026] Weigh 60g of Neolistat, 24g of polyethylene glycol 6000, mix them evenly, add them into the container, heat and melt at a constant temperature of 70°C, stir evenly, remove the heat, add 12g of mannitol, stir evenly, cool to room temperature, take out, and 30 mesh sieves to obtain the granules containing new lisstat; the new lisstat granules and the remaining mannitol, the microcrystalline cellulose of the prescription amount, croscarmellose sodium, iron oxide yellow, magnesium stearate together After mixing evenly, go directly to the machine to compress the tablet, that is to say.
Embodiment 2
[0028] 1) Prescription composition
[0029] prescription composition 500 tablets / g Neolistat 60 polyethylene glycol 6000 48 Mannitol 30 microcrystalline cellulose 7.5 Croscarmellose Sodium 11 Iron oxide yellow 0.2 Magnesium stearate 2.3
[0030] 2) Preparation method
[0031] Weigh 60g of Neolistat, 48g of polyethylene glycol 6000, mix them evenly, add them into the container, heat and melt at a constant temperature of 65°C, stir evenly, remove the heat, add 15g of mannitol, stir evenly, cool to room temperature, take out, and 30 mesh sieves to obtain the granules containing new lisstat; the new lisstat granules and the remaining mannitol, the microcrystalline cellulose of the prescription amount, croscarmellose sodium, iron oxide yellow, magnesium stearate together After mixing evenly, go directly to the machine to compress the tablet, that is to say.
Embodiment 3
[0033] 1) Prescription composition
[0034] prescription composition 500 tablets / g Neolistat 60 polyethylene glycol 6000 48 Mannitol 30 microcrystalline cellulose 7.5 Crospovidone 8 Iron oxide yellow 0.2 Magnesium stearate 2.3
[0035] 2) Preparation method
[0036] Weigh 60g of Neolistat, 48g of polyethylene glycol 6000, mix them evenly, add them into the container, heat and melt at a constant temperature of 65°C, stir evenly, remove the heat, add 15g of mannitol, stir evenly, cool to room temperature, take out, and 30 mesh sieves to obtain the new lisstat granules; after mixing the new lisstat granules with the remaining mannitol, the prescribed amount of microcrystalline cellulose, cross-linked povidone, yellow iron oxide, and magnesium stearate , directly on the machine to compress the tablet, that is to say.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com