A kind of bone filling adhesive and its preparation method and application
An adhesive and adhesive technology, used in surgical adhesives, applications, pharmaceutical formulations, etc., can solve problems such as high brittleness, low mechanical strength, and difficulty in curing blood
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
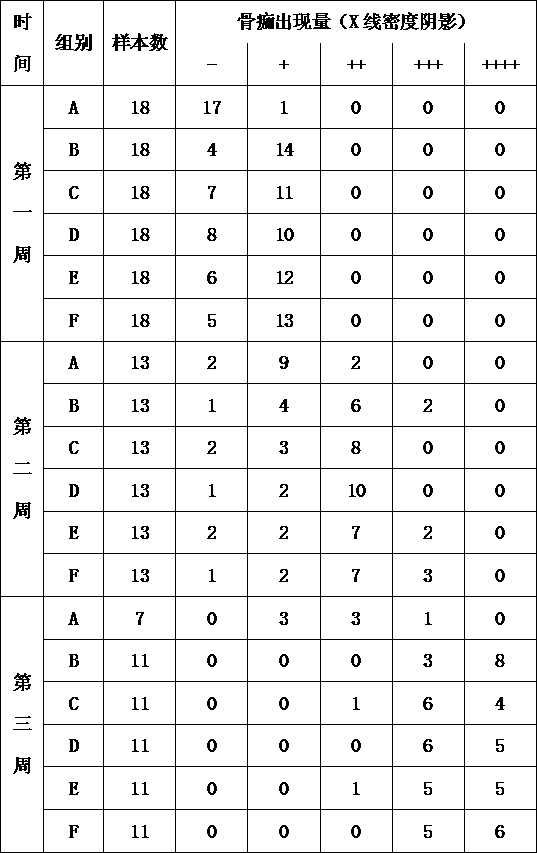
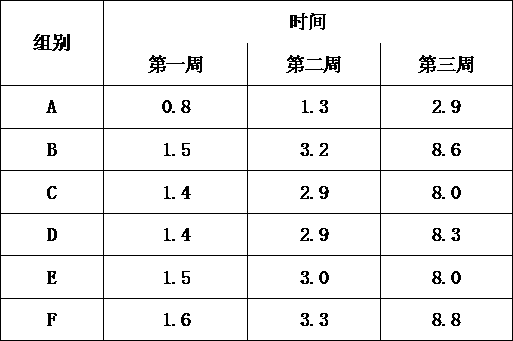
Image
Examples
Embodiment 1
[0037] A bone filling adhesive, which is made of the following raw materials: PLGA: ethyl acetate: bioglue: phosphate buffer: distilled water = 10g ~ 30g: 25ml ~ 60ml: 10ml ~ 20ml: 300ml ~ 600ml: 10ml ~ 50ml,
[0038] Or PLGA: acetone: biological glue: phosphate buffer: distilled water = 10g ~ 30g: 20ml ~ 30ml: 10ml ~ 20ml: 300ml ~ 600ml: 10ml ~ 50ml,
[0039] Or PLGA: dichloromethane: biological glue: phosphate buffer: distilled water = 10g ~ 30g: 20ml ~ 50ml: 10ml ~ 20ml: 300ml ~ 600ml: 10ml ~ 50ml (the ratio of raw materials in this embodiment is PLGA: ethyl acetate : bioglue: phosphate buffer: distilled water=20g: 50ml: 20ml: 450ml: 20ml); the bioglue is a fibrin adhesive.
[0040] The bioglue described in this example is a pig-derived fibrin adhesive.
[0041] A preparation method of the above-mentioned bone filling adhesive, which comprises the following steps:
[0042] (1) Dissolve PLGA in ethyl acetate according to the proportion to obtain solution A (this embodimen...
Embodiment 2
[0054] A bone filling adhesive, which is made of the following raw materials: PLGA: ethyl acetate: bioglue: phosphate buffer: distilled water = 10g ~ 30g: 25ml ~ 60ml: 10ml ~ 20ml: 300ml ~ 600ml: 10ml ~ 50ml,
[0055] Or PLGA: acetone: biological glue: phosphate buffer: distilled water = 10g ~ 30g: 20ml ~ 30ml: 10ml ~ 20ml: 300ml ~ 600ml: 10ml ~ 50ml,
[0056] Or PLGA: dichloromethane: biological glue: phosphate buffer: distilled water = 10g ~ 30g: 20ml ~ 50ml: 10ml ~ 20ml: 300ml ~ 600ml: 10ml ~ 50ml (the ratio of raw materials in this embodiment is PLGA: ethyl acetate : bioglue: phosphate buffer: distilled water=10g: 60ml: 10ml: 400ml: 10ml); the bioglue is a fibrin adhesive.
[0057] A preparation method of the above-mentioned bone filling adhesive, the temperature condition in step (3) of this embodiment is 8°C.
[0058] The raw materials and proportioning ratio of this embodiment are PLGA:ethyl acetate=10g:60ml.
[0059] The ratio described in step (2) of this embodimen...
Embodiment 3
[0064] A bone filling adhesive, which is made of the following raw materials: PLGA: ethyl acetate: bioglue: phosphate buffer: distilled water = 10g ~ 30g: 25ml ~ 60ml: 10ml ~ 20ml: 300ml ~ 600ml: 10ml ~ 50ml,
[0065] Or PLGA: acetone: biological glue: phosphate buffer: distilled water = 10g ~ 30g: 20ml ~ 30ml: 10ml ~ 20ml: 300ml ~ 600ml: 10ml ~ 50ml,
[0066] Or PLGA: dichloromethane: biological glue: phosphate buffer: distilled water = 10g ~ 30g: 20ml ~ 50ml: 10ml ~ 20ml: 300ml ~ 600ml: 10ml ~ 50ml (the ratio of raw materials in this embodiment is PLGA: ethyl acetate : bioglue: phosphate buffer: distilled water=30g: 45ml: 15ml: 600ml: 50ml); the bioglue is a fibrin adhesive.
[0067] A preparation method of the above-mentioned bone filling adhesive, the raw materials and the proportion of this embodiment are PLGA:ethyl acetate=30g:45ml.
[0068] The ratio described in step (2) of this embodiment is biological glue: phosphate buffer solution = 15ml: 600ml.
[0069] An appl...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com