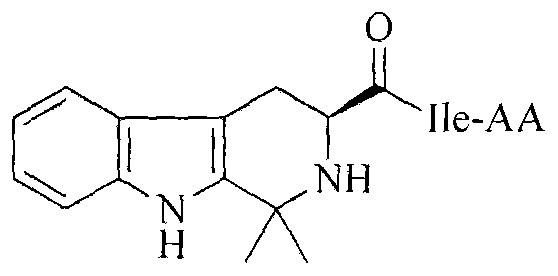
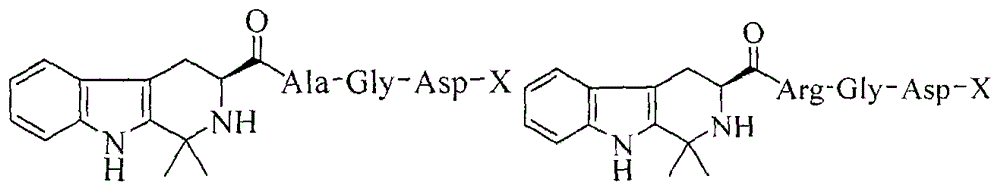Dimethyl tetrahydrocarboline-3-formyl-Ile-AA, and synthesis, activity and application thereof
A technology of dimethyl and carboline, applied in the field of biomedicine
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
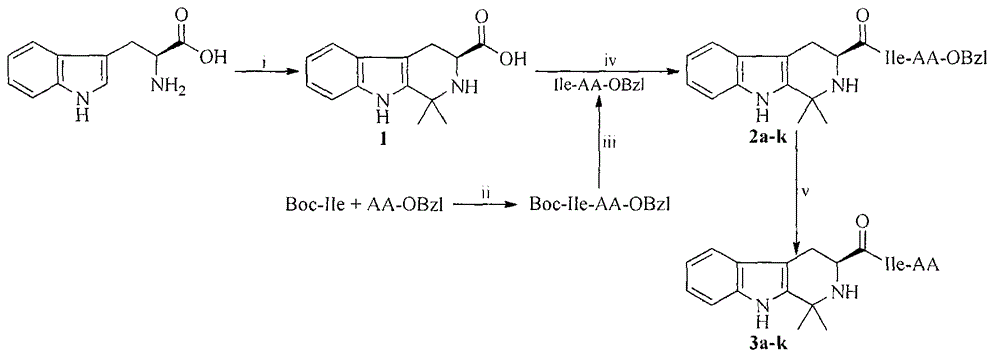
[0017] Example 1 Preparation of (3s)-1,1-dimethyl-tetrahydro-β-carboline-3-carboxylic acid (1)
[0018] Weigh 4.08g (20mmol) of L-Trp into a 250mL eggplant bottle, and slowly add 80mL of acetone. Under ice-bath stirring conditions, 4 mL of concentrated hydrochloric acid was added to the suspension reaction solution, and then 2 mL of distilled water was added, and after stirring for 0.5 h, the ice-bath was removed for 24 h. After 24h, the raw material spot monitored by thin layer chromatography disappeared. The hydrogen chloride gas in the reaction solution was pumped out by a water pump and the reaction solution was concentrated. After adding 100 mL of ether, it was allowed to stand still to obtain 5.22 g (93%) of the title compound as colorless crystals. ESI-MS(m / e)245.1212[M+H] + .
Embodiment 2
[0019] Example 2 Preparation of (3s)-1,1-dimethyl-tetrahydro-β-carboline-3-formyl-Ile-Ala-OBzl(2a)
[0020] Under ice bath and stirring, add 135mg (1mmol) N-hydroxyl to a solution of 281mg (1mmol) (3s)-1,1-dimethyl-tetrahydro-β-carboline-3-carboxylic acid and 20mL anhydrous tetrahydrofuran Benzotriazole, 207 mg (1 mmol) of dicyclohexylcarbonyl diimide and 281 mg (1 mmol) of Ile-Ala-OBzl, the pH of the reaction solution was adjusted to 9 with N-methylmorpholine. The reaction was carried out in an ice bath for 0.5h, and then it was reacted at room temperature until TLC (petroleum ether:acetone, 2:1) to monitor the completion of the reaction. The generated dicyclohexylurea was removed by filtration, the filtrate was concentrated under reduced pressure, and the residue obtained was dissolved in 30 mL of ethyl acetate. The ethyl acetate solution was washed 3 times with saturated sodium bicarbonate aqueous solution and 3 times with saturated sodium chloride aqueous solution. % Potassi...
Embodiment 3
[0021] Example 3 Preparation of (3s)-1,1-dimethyl-tetrahydro-β-carboline-3-formyl-Ile-Asp(OBzl)-OBzl(2b)
[0022] According to the method of Example 2 from 1.12g (4mmol) (3s)-1,1-dimethyl-tetrahydro-β-carboline-3-carboxylic acid and 1.70g (4mmol) Ile-Asp(OBzl)-OBzl This gave 681 mg (26%) of the title compound as a pale yellow solid. Mp: 91-92°C; ESI-MS(m / e)653[M+H] + ; 1 H-NMR(300MHz, CDCl 3 ): δ / ppm = 7.80 (s, 1H), 7.62 (d, J = 9.0 Hz, 1H), 7.51 (d, J = 6.0 Hz, 1H), 7.35 (m, 10H), 7.15 (m, 2H) , 6.90 (d, J = 9.0 Hz, 1H), 5.13 (dd, J = 3.0, 18.0 Hz, 4H), 4.99 (m, 1H), 4.39 (dd, J = 6.0, 9.0 Hz, 1H), 3.76 ( dd, J = 6.0, 12.0 Hz, 2H), 3.31 (dd, J = 6.0, 15.0 Hz, 1H), 3.02 (m, 2H), 2.68 (dd, J = 12.0, 15.0 Hz, 1H), 1.95 (m , 1H), 1.61 (m, 2H), 1.54 (s, 3H), 1.47 (s, 3H), 1.20 (m, 2H), 0.95 (m, 6H).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com