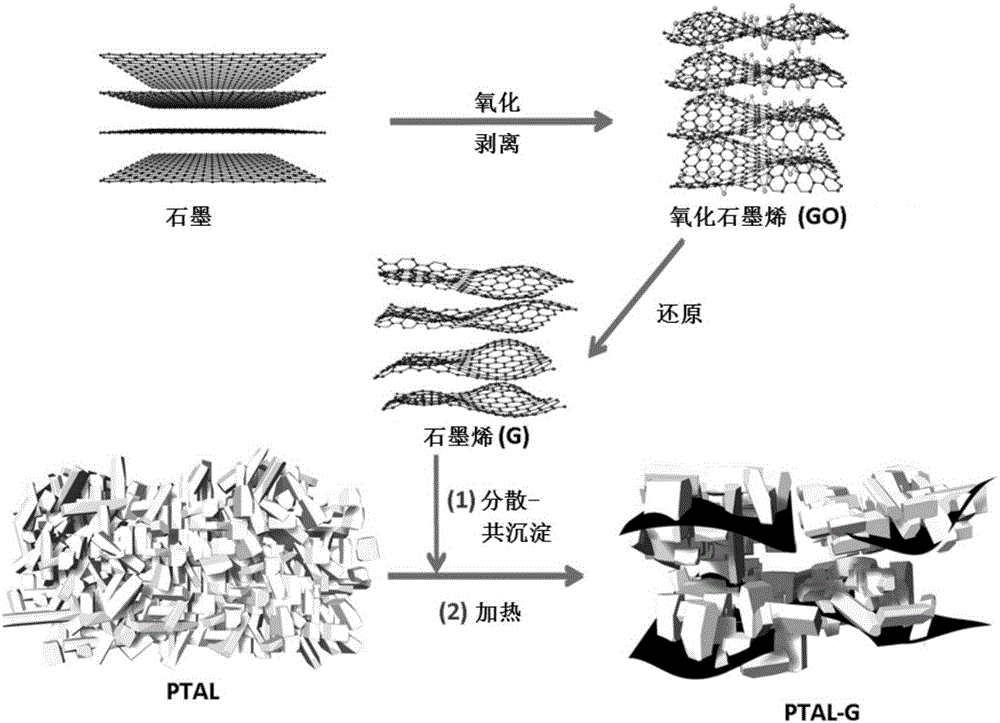
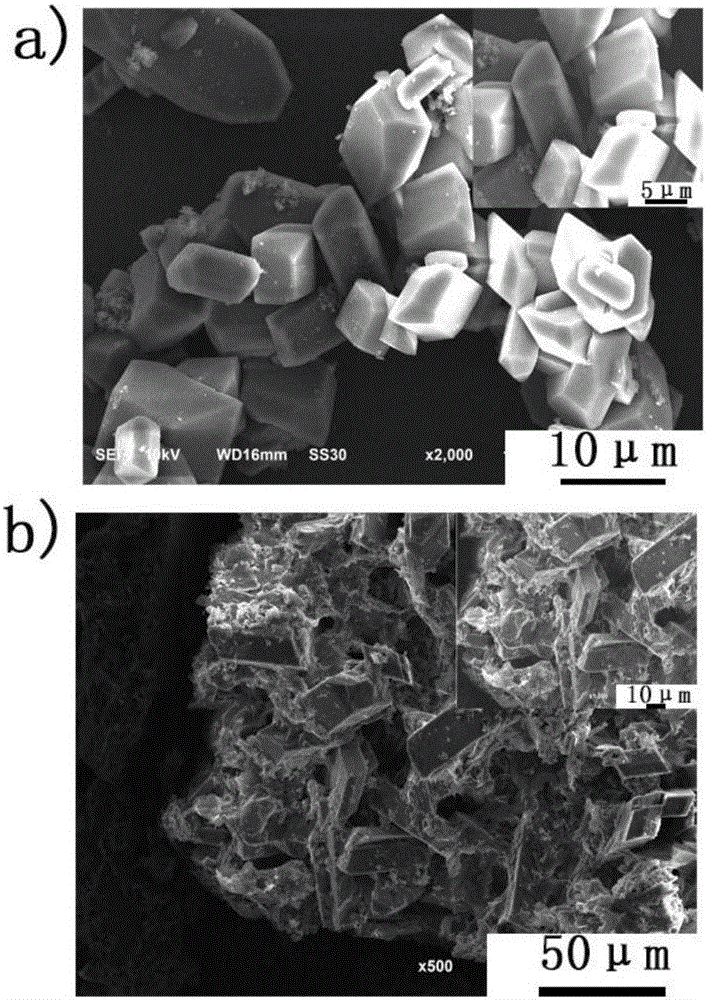
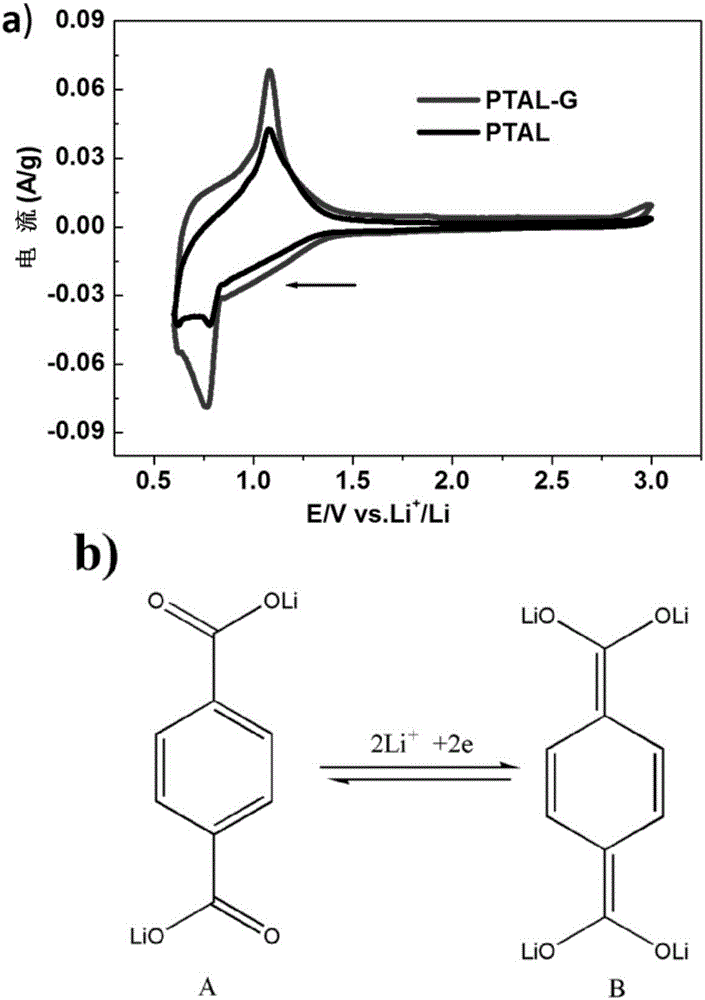
Lithium terephthalate-graphene compound as well as preparation and application thereof
A technology of lithium terephthalate and terephthalic acid, which is applied in the field of lithium terephthalate-graphene composites and its preparation and application, can solve the problems of reversible capacity fading and poor cycle performance, and achieve avoidance of polymerization, The effect of excellent electrochemical performance and simple preparation method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0031] (1) Add 0.85g LiOH·H 2 O was dissolved in 25 mL of deionized water, 1.66 g of terephthalic acid was dissolved in 50 mL of ethanol, and then stirred at 70 °C, the terephthalic acid solution was gradually added dropwise to the LiOH solution. After continuing to reflux and stir for 8 hours to stop heating, the solution was centrifuged, and the obtained precipitate was washed with ethanol and deionized water with a volume ratio of 1:1, and then dried in a vacuum oven at 70°C for 12 hours to obtain white lithium terephthalate (PTAL ).
[0032] (2) 0.4g of natural graphite powder and 0.35g of NaNO 3 into a three-neck flask with a stirrer chip, then slowly drop 30mL of 98% H 2 SO 4 . The mixture was stirred under ice-water bath for 1 hour. Then, 1.8 g of potassium permanganate (purity 99%) was gradually added and stirred slowly for 3 hours. The thus formed mixture was reacted at room temperature for seven days. Subsequently, 40 mL of 5% H 2 SO 4 The aqueous solution w...
Embodiment 2
[0042] (1) 0.90g LiOH·H 2 O was dissolved in 25 mL of deionized water, 1.66 g of terephthalic acid was dissolved in 50 mL of methanol, and then stirred at 60 °C, the terephthalic acid solution was gradually added dropwise to the LiOH solution. Continue to reflux and stir for 8 hours, stop heating, centrifuge the solution, wash the obtained precipitate with methanol and deionized water with a volume ratio of 1:1, and then dry it in a vacuum oven at 60°C for 12 hours to obtain white lithium terephthalate.
[0043] (2) 0.4g of natural graphite powder and 0.35g of NaNO 3 into a three-neck flask with a stirrer chip, then slowly drop 30mL of 98% H 2 SO 4 . The mixture was stirred under ice-water bath for 1 hour. Then, 1.8 g of potassium permanganate (purity 99%) was gradually added and stirred slowly for 3 hours. The thus formed mixture was reacted at room temperature for seven days. Subsequently, 40 mL of 5% H 2 SO 4 The aqueous solution was added to the solution and stirre...
Embodiment 3
[0047] (1) 0.95g LiOH·H 2 O was dissolved in 25 mL of deionized water, 1.66 g of terephthalic acid was dissolved in 50 mL of N,N-dimethylformamide, and then stirred at 80 °C, the terephthalic acid solution was gradually added dropwise to the LiOH solution. Continue to reflux and stir for 8 hours, stop heating, centrifuge the solution, wash the obtained precipitate with N,N-dimethylformamide and deionized water with a volume ratio of 1:1, and then dry it in a vacuum oven at 80°C for 12 hours to obtain a white of lithium terephthalate.
[0048] (2) 0.4g of natural graphite powder and 0.35g of NaNO 3 into a three-neck flask with a stirrer chip, then slowly drop 30mL of 98% H 2 SO 4 . The mixture was stirred under ice-water bath for 1 hour. Then, 1.8 g of potassium permanganate (purity 99%) was gradually added and stirred slowly for 3 hours. The thus formed mixture was reacted at room temperature for seven days. Subsequently, 40 mL of 5% H 2 SO 4 The aqueous solution was ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com