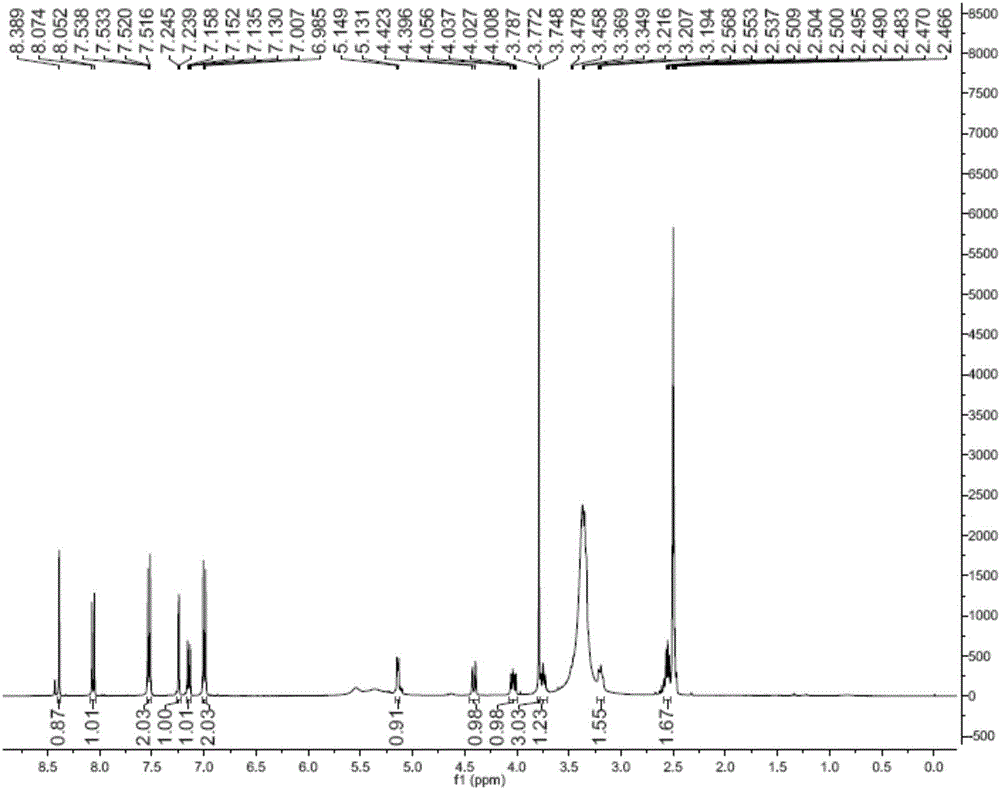
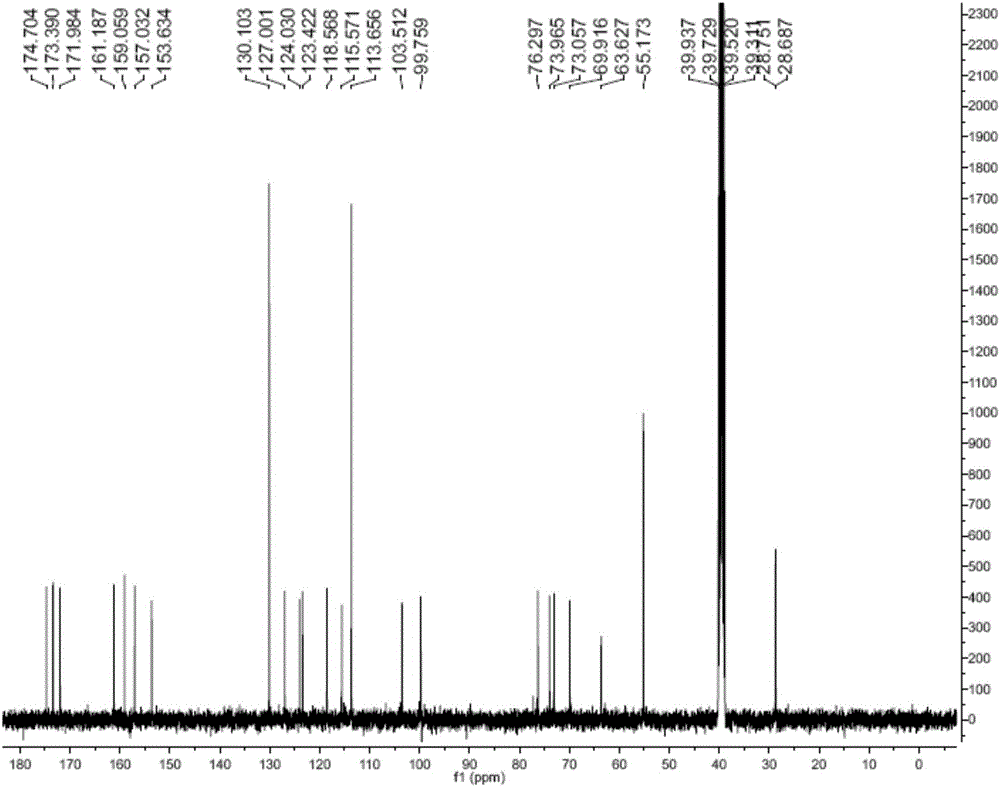
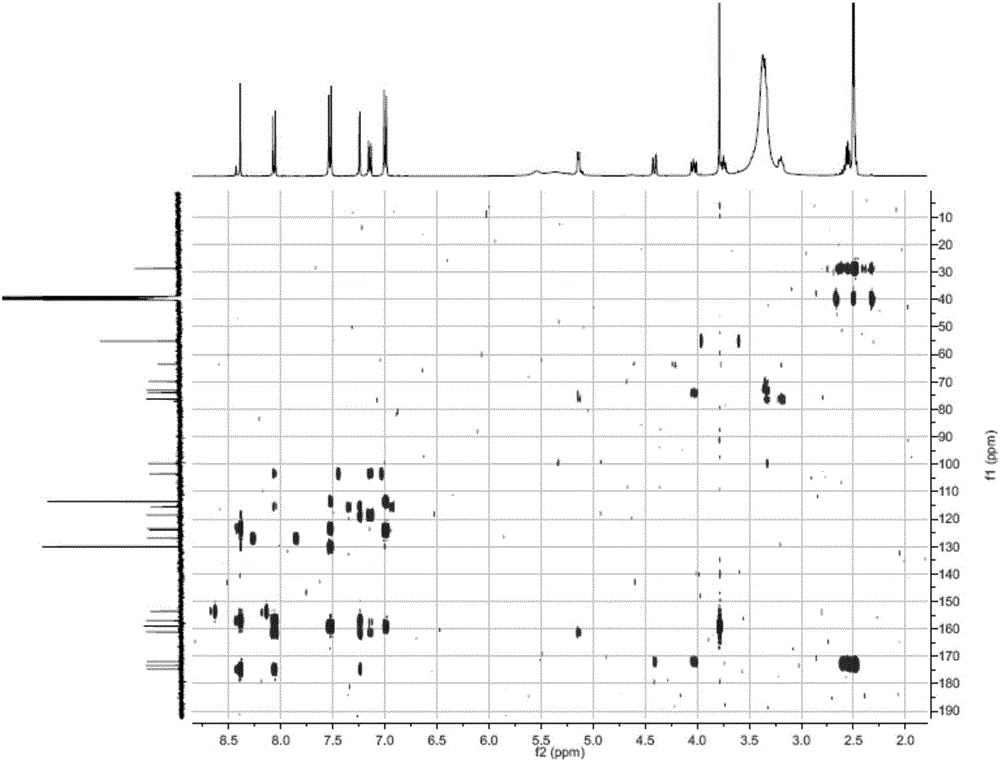
Succinyl ononin and applications of succinyl ononin in preparation of cardiovascular disease medicine
A succinyl formonioides and cardiovascular technology, applied in the field of medicine, can solve the problems of high structural toxicity, high cost, complicated purification steps and the like, and achieve the effect of high water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Screening and identification of strains producing succinylformononetin in non-aqueous phase
[0034] Take about 1g of soil sample, add it to the Erlenmeyer flask containing 20mL of screening medium, 30℃, 180r·min -1 The cells were incubated with shaking in a water bath for 24 hours, and then transferred 3 times with 5% inoculum. After appropriate dilution of the culture liquid, it was diluted and spread with LB plate medium, cultured at 30°C for 24 hours, the colony morphology was observed, and then single colonies were picked and streaked on the LB plate medium to obtain single clones, and the strains obtained by screening were screened. numbered and cryopreserved at ultra-low temperature.
[0035] Using sucrose as the growth carbon source of organic solvent-resistant bacteria and adding 20% (v / v) dimethylformyl as the screening pressure, organic solvent-resistant extremophiles were obtained by screening and separating from chemically polluted soil samples.
[0036...
Embodiment 2
[0041] Fermentation of Bacillus amyloliquefaciens FJ18 and Preparation of Resting Cells
[0042] The fermentation of Bacillus amyloliquefaciens FJ18 was inoculated into the seed medium: yeast extract 5.0 g / L, peptone 10.0 g / L, NaCl 10.0 g / L, pH 7.0, cultured at 30° C., 200 rpm for 12 hours. Expansion medium and fermentation medium, whose components and contents are: sucrose 20g / L, yeast powder 15g / L.KH 2 PO 4 1.0g / L, CaCl 2 0.8g / L. The pH was adjusted to 8.0 with NaOH. The seed liquid was inoculated into expansion medium and fermentation medium at 0.5% (v / v), and cultured at 30° C. and 200 rpm for 12 hours. After centrifugation at 10,000 rpm for 15 minutes, the bacterial cells were collected and washed with physiological saline for 1-2 times to obtain the resting cells of Bacillus amyloliquefaciens FJ18.
Embodiment 3
[0044] The bacterial cell fermentation broth in Example 2 was filtered to obtain wet bacterial cells. The raw material solution, namely the reaction solution, is prepared with dimethyl sulfoxide, formononetin, sucrose and phosphate buffer. The ratio of organic solvent dimethyl sulfoxide in the reaction solution is 20% (v / v), formononetin 0.5g / L, the molar concentration of phosphate buffer is 150mmol / L, the pH of phosphate buffer is 8.0, and the sucrose concentration is 50g / L. The wet cells obtained above were dispersed in the reaction solution, added to the reactor, cultured at 30°C and 200rpm for 24h, centrifuged at 10,000rpm for 10 minutes to obtain the supernatant of the reaction solution, and the HPLC detection and analysis detected the conversion of formononetin. The rate was 97.2%.
[0045] The product was separated with macroporous resin, and an appropriate amount of resin was taken and soaked in ethanol for 24 hours to remove resin fragments and debris. wet packing...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com