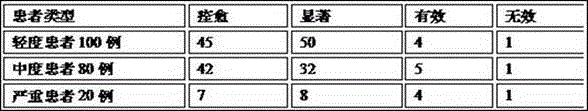
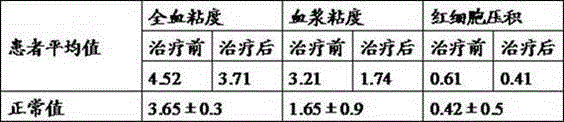
Medical applications and administration route for ozonized oil
A technology of ozonated oil and drug delivery route, which is applied in the direction of medical preparations containing active ingredients, pharmaceutical formulas, digestive system, etc., and can solve the problems of unclear specific mechanism, long course of treatment, difficulty in ensuring the effective concentration of gas entering the body, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1 Preparation of ozonated olive oil enteric-coated capsules
[0037] 1. Preparation of ozone gas. The invention adopts medical oxygen to prepare ozone gas through an ozone generator.
[0038] It is selected from the DRO3-A ozone machine (power≦55W) designed and produced by Xi'an Derun Biotechnology Co., Ltd.
[0039] 2. The whole process of preparing ozonated oil and ozonated oil enteric-coated capsules must be carried out at 20-25°C to ensure that the ozonated oil does not undergo pyrolysis.
[0040] 3. Put 3kg of olive oil into the reactor; 3 / h flow rate was added, and the total reaction time was 20 hours. After the reaction is over, the residual gas is inactivated.
[0041] 4. The obtained ozonated olive oil has a concentration of 40 mg / L and can be stored stably for ten months at 25°C.
[0042] 5. Preparation of ozonated olive oil enteric-coated capsules.
[0043] (1) Preparation of capsule material: Weigh water, gelatin, glycerin and glutinous rice f...
Embodiment 2
[0046] Embodiment 2 Preparation of enteric-coated capsules of ozonized ozonated olive oil, soybean oil and peanut oil mixed oil
[0047] 1. Preparation of ozone gas. The invention adopts medical oxygen to prepare ozone gas through an ozone generator.
[0048] It is selected from the DRO3-A ozone machine (power≦55W) designed and produced by Xi'an Derun Biotechnology Co., Ltd.
[0049] 2. The whole process of preparing ozonated oil and ozonated oil enteric-coated capsules must be carried out at 20-25°C to ensure that the ozonated oil does not undergo pyrolysis.
[0050] 3. Put 1kg of olive oil, 1kg of soybean oil and 1kg of peanut oil into the reaction kettle; 3 / h flow rate was added, and the total reaction time was 20 hours. After the reaction is over, the residual gas is inactivated.
[0051] 4. The concentration of the obtained ozonated mixed oil is 40mg / L, and it can be stored stably for ten months at 25°C.
[0052] 5. Preparation of ozonated mixed oil enteric-coated c...
Embodiment 3
[0056] Example 3 Preparation of ozonated olive oil enteric-coated capsules containing surfactant
[0057] 1. Preparation of ozone gas. The invention adopts medical oxygen to prepare ozone gas through an ozone generator.
[0058] It is selected from the DRO3-A ozone machine (power≦55W) designed and produced by Xi'an Derun Biotechnology Co., Ltd.
[0059] 2. The whole process of preparing ozonated oil and ozonated oil enteric-coated capsules must be carried out at 20-25°C to ensure that the ozonated oil does not undergo pyrolysis.
[0060] 3. Put 3kg of olive oil into the reaction kettle, slowly add 20mL of sorbitan monooleate polyoxyethylene ether (Tween 80), mix well, and use ozone at 150m 3 / h flow rate was added, and the total reaction time was 20 hours. After the reaction is over, the residual gas is inactivated.
[0061] 4. The obtained ozonated olive oil has a concentration of 40 mg / L and can be stored stably for ten months at 25°C.
[0062] 5. Preparation of ozonate...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com