Monoclonal antibody for recognizing HPV16 positive tumor cells, and applications thereof
An antibody and carrier technology, applied in applications, antibodies, anti-tumor drugs, etc., can solve problems such as low sensitivity, inability to obtain immune response, and lack of specificity of E7 protein
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0175] Preparation of monoclonal antibodies
[0176] Antibodies of the present invention can be prepared by various techniques known to those skilled in the art. For example, an antigen of the invention may be administered to an animal to induce the production of monoclonal antibodies. For monoclonal antibodies, hybridoma technology can be used to prepare (see Kohler et al., Nature 256; 495, 1975; Kohler et al., Eur.J.Immunol.6:511, 1976; Kohler et al., Eur.J.Immunol. 6:292,1976; Hammerling et al., In Monoclonal Antibodies and T Cell Hybridomas, Elsevier, N.Y., 1981), phage display technology or available recombinant DNA method (US Patent No. 4,816,567).
[0177]Representative myeloma cells are those that fuse efficiently, support stable high-level production of antibody by selected antibody-producing cells, and are sensitive to culture medium (HAT medium matrix), including myeloma cell lines, such as murine Myeloma cell lines, including those derived from MOPC-21 and MPC-11...
Embodiment 1
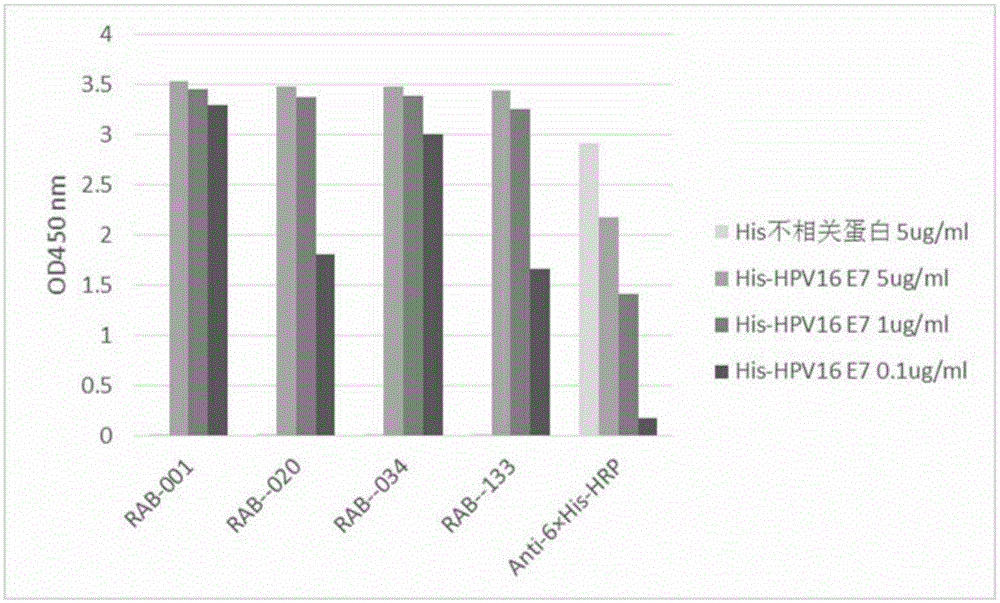
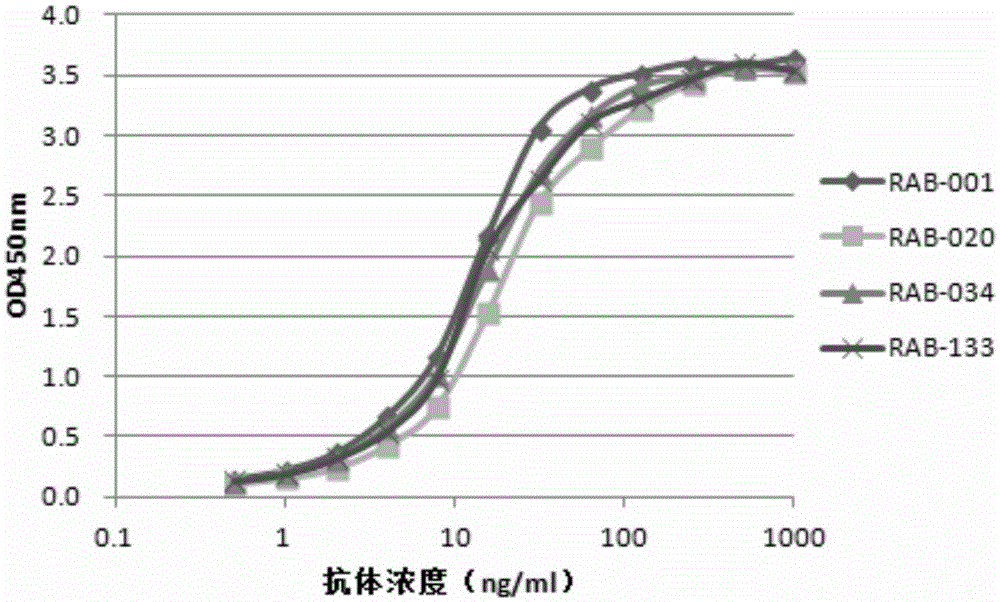
[0212] 1. Preparation of rabbit monoclonal antibody against human papillomavirus HPV16 E7
[0213] 1.1 Screening of single-chain antibody (scFv)
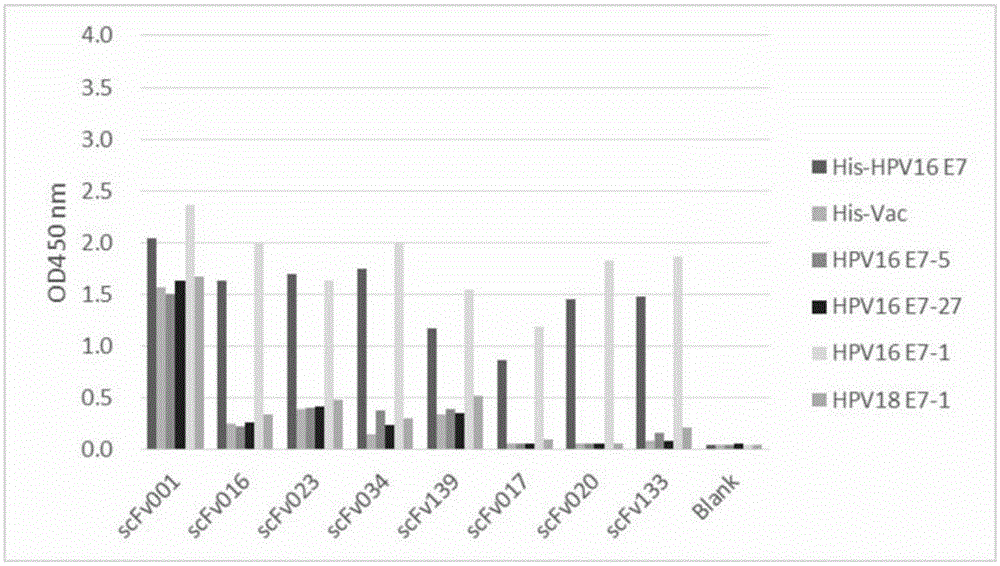
[0214] Rabbits were immunized with His-HPV16 E7 recombinant protein, and the titer was detected with His-HPV16 E7 recombinant protein and His irrelevant protein. Isolation of rabbit B lymphocytes to obtain immunoglobulin genes. The complete set of variable region genes of B cells is cloned and assembled into a phage antibody library. The constructed phage antibody library was panned with the recombinant protein His-HPV16 E7. Enrichment after three rounds of panning; determination of phage titer; amplification of phage plaques; DNA sequencing; The recombinant protein His-HPV16 E7 was selected for ELISA detection and screening, and the negative control (N) was set with His irrelevant protein, the Anti-6×His antibody was set as the positive control (P) coated with His antigen, and the blank control (coated with the antigen directly)...
Embodiment 2
[0235] Example 2 Detection of overexpression of biomarkers in tumor cells treated with liquid-based cell fixative using immunocytochemical staining
[0236] Collect CaSki cells and C-33A cells in the logarithmic growth phase by centrifugation, mix Caski cells and C-33A cells at a ratio of 1:1, and plant them on poly-L-Lysine-treated coverslips at 37°C, 5% CO 2 Cultivate for 24h. Carefully rinse twice with PBS after discarding the medium. The coverslip was taken out, and liquid-based cell fixative (Hologic, Preserv Solution) fixed solution for several days. Before use, place the coverslip in 99% ethanol for 10 min to 1 h, and air-dry overnight.
[0237] After the air-dried tumor cell coverslips were placed in 50% ethanol for 10 min, they were transferred to deionized water for at least 30 s. To prevent non-specific background staining, do not allow coverslips to dry out during staining. Place the deionized water-treated cell slides in Tris-EDTA (pH 9.0) repair solution, ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com