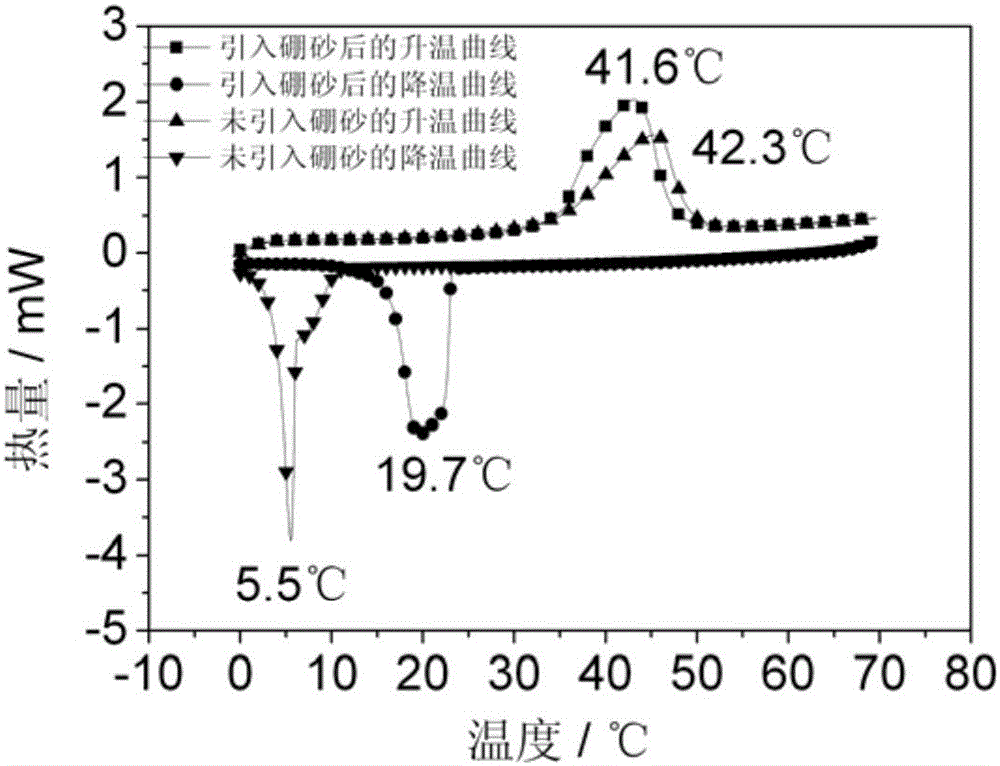
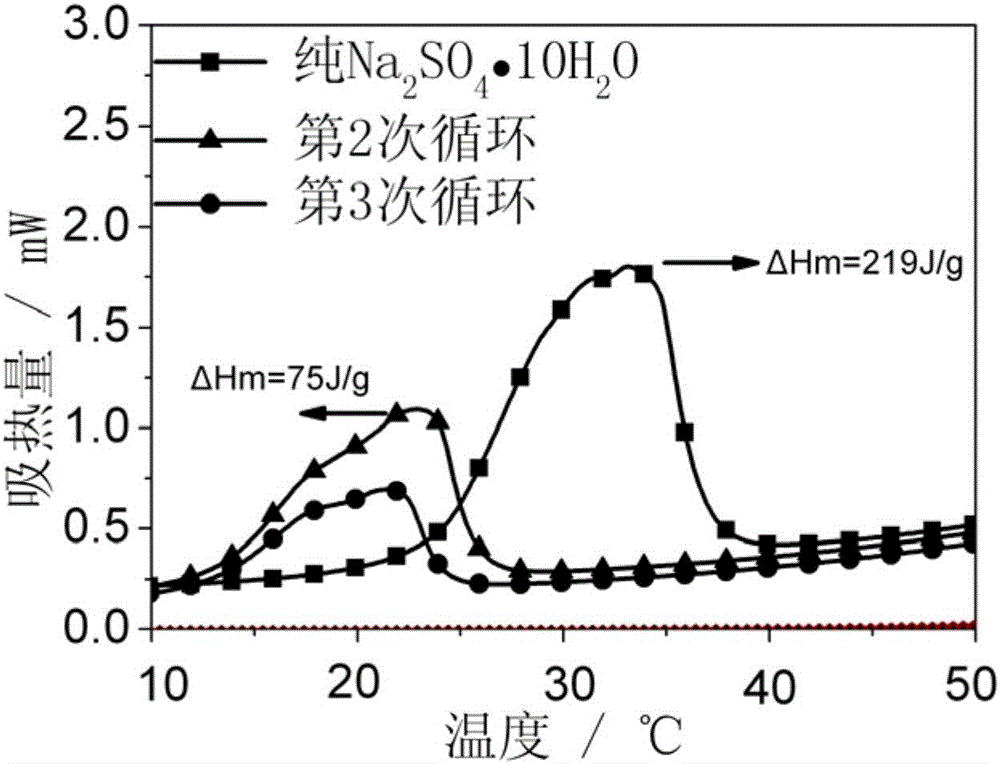
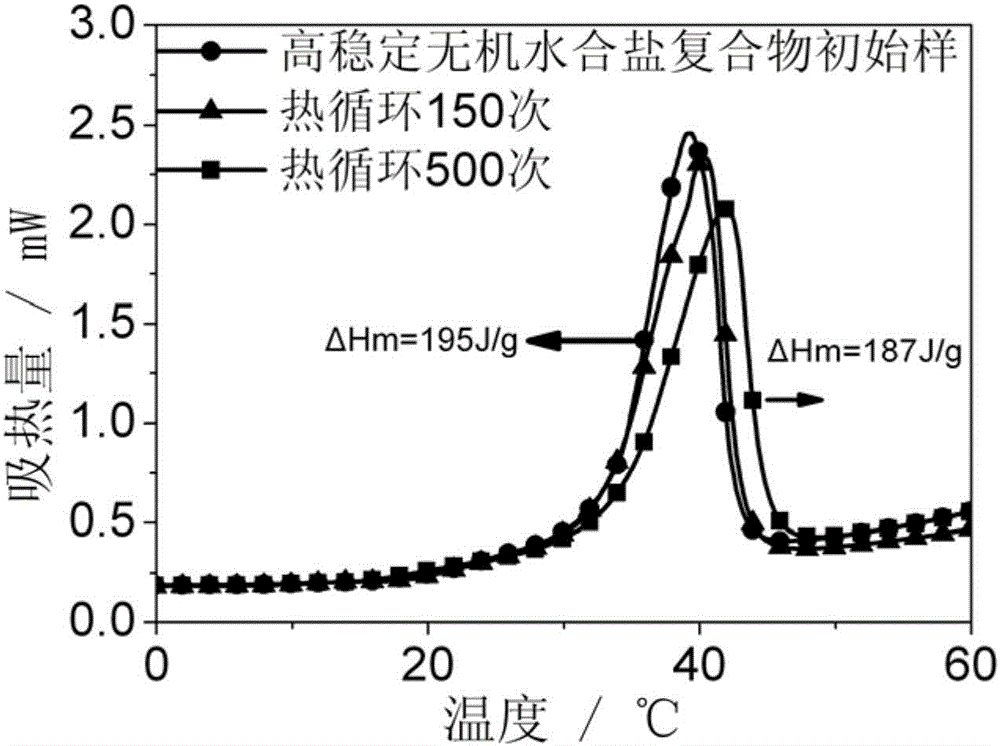
High-stability composite phase-change material and preparation method
A composite phase change material, high stability technology, applied in heat exchange materials, chemical instruments and methods, etc., can solve problems such as cycle stability deterioration, and achieve the effects of stable performance, improved stability, and less equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] 1) Weigh Na 2 SO 4 10H 2 O 6 kilograms of inorganic hydrated salts were placed in a stirred tank and the temperature was raised to 40° C., until the inorganic hydrated salts became molten.
[0031] 2) In molten Na 2 SO 4 10H 2 Add 0.18 kilograms of borax in O inorganic hydrated salt. Keep the temperature at 40°C, continue stirring for 60 minutes, then lower the temperature and cool to room temperature to obtain a mixed salt. The mixed salt is ball-milled to 100 mesh in a ball mill, and sieved for later use.
[0032] 3) Weigh 3.7 kg of polyacrylamide, add distilled water, and stir evenly in the stirring tank to form a viscosity of 5×10 4 centipoise polymer colloid. Put in the mixed salt described in step 2 and stir well.
[0033] 4) Transfer the material obtained in step 3 to a kneading pot, and knead at room temperature for 0.5 hours. During the kneading process, the two knife rollers cut, pulled, and extruded the compound of inorganic hydrated salt / polymer co...
Embodiment 2
[0035] 1) Weigh Na 2 SO 410H 2 O 9.4 kilograms of inorganic hydrated salts were placed in a stirred tank and the temperature was raised to 45° C., until the inorganic hydrated salts became molten.
[0036] 2) In molten Na 2 SO 4 10H 2 Add 0.1 kg of borax to the inorganic hydrated salt, keep the temperature at 45° C., continue to stir for 50 minutes, then lower the temperature and cool to room temperature to obtain a mixed salt. The mixed salt is ball-milled to 150 mesh in a ball mill, and sieved for later use.
[0037] 3) Drop into 5 kilograms of acrylic acid amide / sodium acrylate binary copolymers in the stirred tank. Add distilled water to form a viscosity of 8×10 6 Colloids in centipoise. Put the mixed salt obtained in step 2 into the colloid, and stir evenly.
[0038] 4) Transfer the materials in step 3 to a kneading pot, heat the kneading pot to 40° C., and knead at this temperature for 2 hours. During the kneading process, part of the free water of the inorgani...
Embodiment 3
[0040] 1) Weigh Na 2 SO 4 10H 2 O 6.5 kilograms of inorganic hydrated salts were placed in a stirred tank and the temperature was raised to 40° C., until the inorganic hydrated salts became molten.
[0041] 2) In molten Na 2 SO 4 10H 2 Add 0.13 kg of borax to the O inorganic hydrated salt, keep the temperature of 40° C., continue to stir for 1 hour, then lower the temperature and cool to room temperature to obtain a mixed salt. Grind the mixed salt in a ball mill to 200 mesh, sieve and set aside
[0042] 3) Put 3.37 kg of carboxyethyl cellulose into the stirring tank, add distilled water, and stir for 30 minutes to form a viscosity of 1 × 10 5 centipoise jelly. Put in the mixed salt described in step 2 and stir well.
[0043] 4) Transfer the material in step 3 to a kneading pot, and knead at 30° C. for 0.6 hours. During the kneading process, the two knife rollers shear, pull and extrude the compound of the inorganic hydrated salt / polymer colloid from opposite and oppo...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Latent heat of phase change | aaaaa | aaaaa |
| Latent heat of phase change | aaaaa | aaaaa |
| Latent heat of phase change | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com