A pharmaceutical composition for treating cancer, especially liver cancer
A technology for the treatment of liver cancer and drugs, applied in drug combinations, pharmaceutical formulations, anti-tumor drugs, etc., can solve the problems of no other diseases, such as expanded application reports of cancer, and achieve good application prospects and value, and the effect of good anti-liver cancer activity.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Example 1: Cytotoxicity GI on different cell lines 50
[0035] Experimental procedure: Miapaca-2 used herein is pancreatic cancer cells, Hela is human cervical cancer cells and Hep3B is human liver cancer cells. Each cell was grown in DMEM culture medium containing 10% fetal bovine serum by mass percentage, the antibiotic concentration in the culture medium was 100 μ / ml penicillin and 100 μg / ml streptomycin, and the cell culture was carried out at a temperature of 37° C. carried out in an incubator with a volume concentration of 5%.
[0036] Cells were evenly plated on a 96-well plate at a density of approximately 5×10 3 , add 200 μl complete medium to each well, and culture overnight. After the cells adhere to the wall and grow smoothly, add serum-free DMEM medium containing 25 μM drug and treat for 24 hours. The PBS (phosphate buffer) solution of MTT was prepared so that the concentration of MTT was 5 μg / m, added to serum-free medium and incubated at 37° C. for 4 ...
Embodiment 2
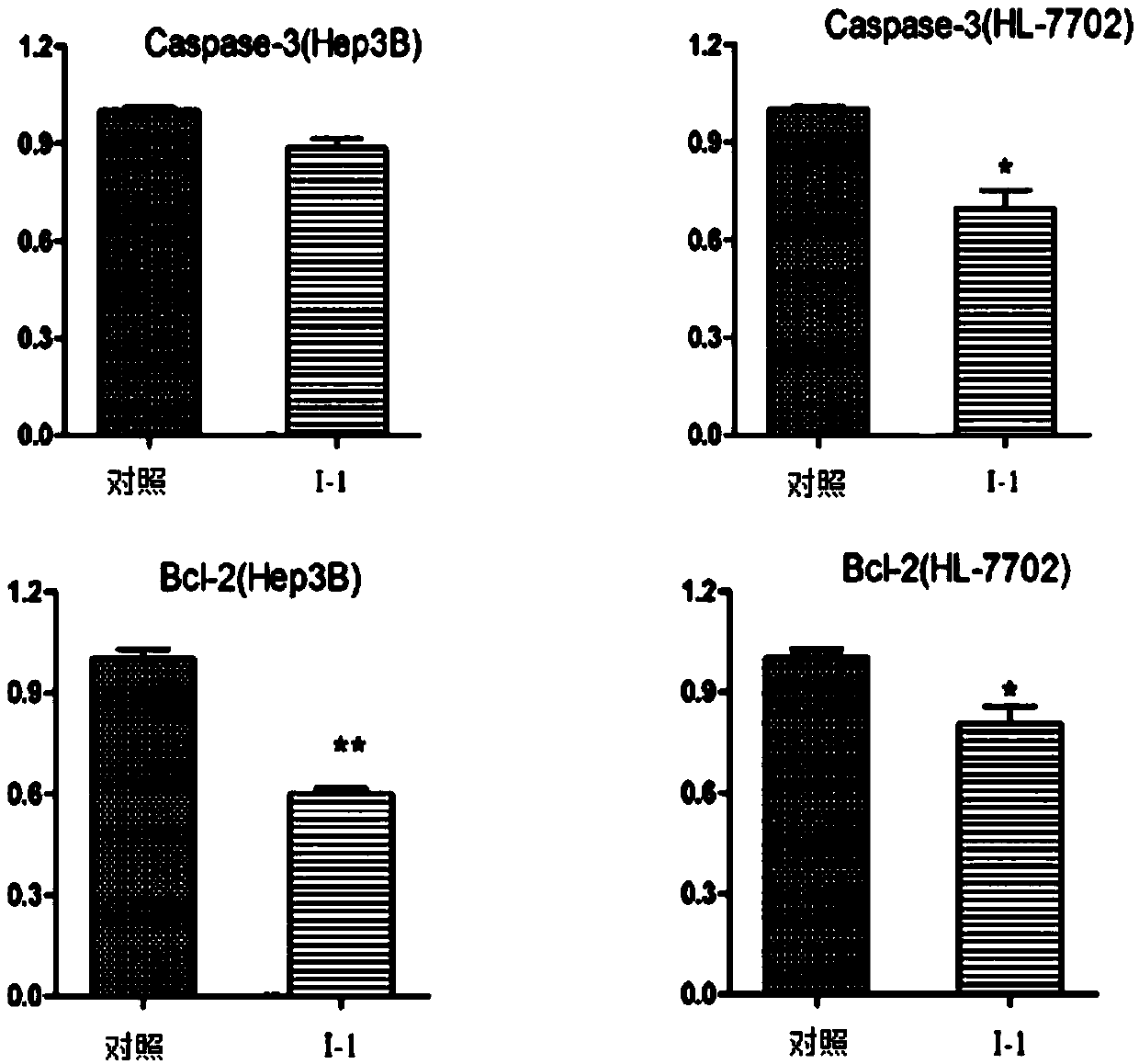
[0041] Example 2: Effects on HL-7702 and Hep3B cell apoptosis and related protein expression
[0042] Experimental procedure: the HL-7702 used here are human normal hepatocytes, and the culture conditions are consistent with the culture of Hep3B mentioned above.
[0043] Hep3B and HL-7702 cells were treated with compounds for 24 hours, trypsinized and harvested. Rinse three times with pre-cooled PBS, add 100 μl cell lysate RIPA and lyse on ice for 30 minutes, vortex from time to time. After centrifugation at 4°C, the supernatant was collected and the protein concentration was determined by the Bradford method. After calculating the appropriate loading concentration, add the protein sample to an appropriate ratio of loading buffer and denature at high temperature for 5 minutes. A polyacrylamide gel with a mass content of 10% of the separating gel and 5% of the stacking gel was configured, and the sample was loaded and run with a current of 40mA and a time of 2 hours. The pro...
Embodiment 3
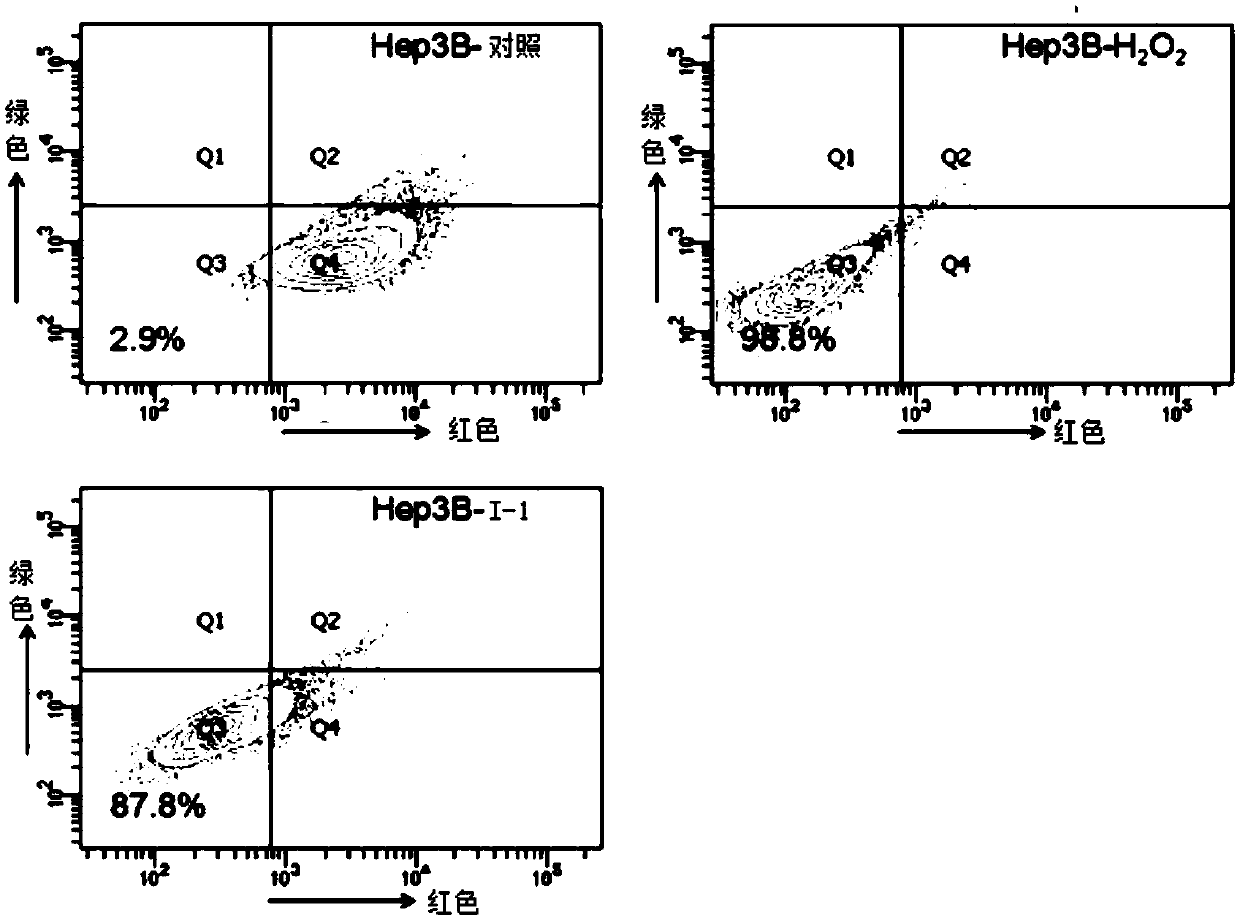
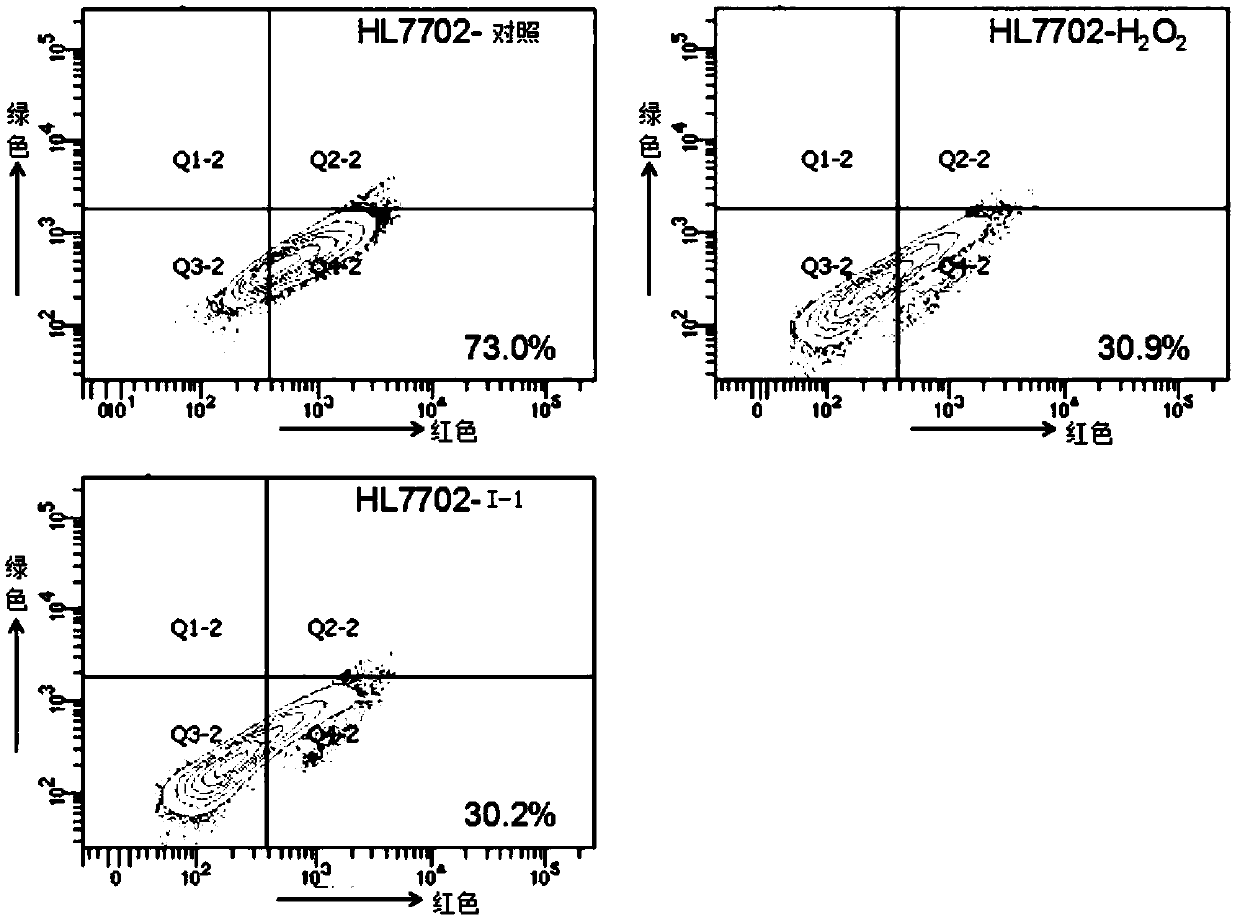
[0047] Example 3: Effects on Mitochondrial Membrane Potential and Voltage-Dependent Anion Channel 1 (VDAC1) Protein in HL-7702 and Hep3B Cells
[0048] Experimental procedure: the mitochondrial membrane potential (MMP) of the cells was measured using the JC-1 cell apoptosis detection kit. JC=1, as a specific fluorescent probe for mitochondrial membrane potential, has two forms. It is a monomer at a low potential and emits green fluorescence; it aggregates into a complex at a high potential and emits red fluorescence. The complete mitochondrial membrane potential is at a high potential, and once the electron chain is damaged, that is, cell apoptosis occurs, the mitochondrial membrane potential will decrease. Fluorescence changes of JC-1 can be considered as an indicator of mitochondrial membrane status.
[0049] After HL-7702 cells and Hep3B cells were treated with drugs for 24 hours, the cells were trypsinized and collected. Rinse twice with PBS, incubate with JC-1 dye worki...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com