A kind of chitosan oligosaccharide with specific structure and its preparation method and application
A chitosan oligosaccharide and a specific technology, applied in the field of chitosan oligosaccharide and its preparation, can solve problems such as differences in biological activity of chitosan oligosaccharides, differences in substrate recognition, etc., and achieve the effects of good pharmacological activity and inhibiting the growth of liver cancer cells
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
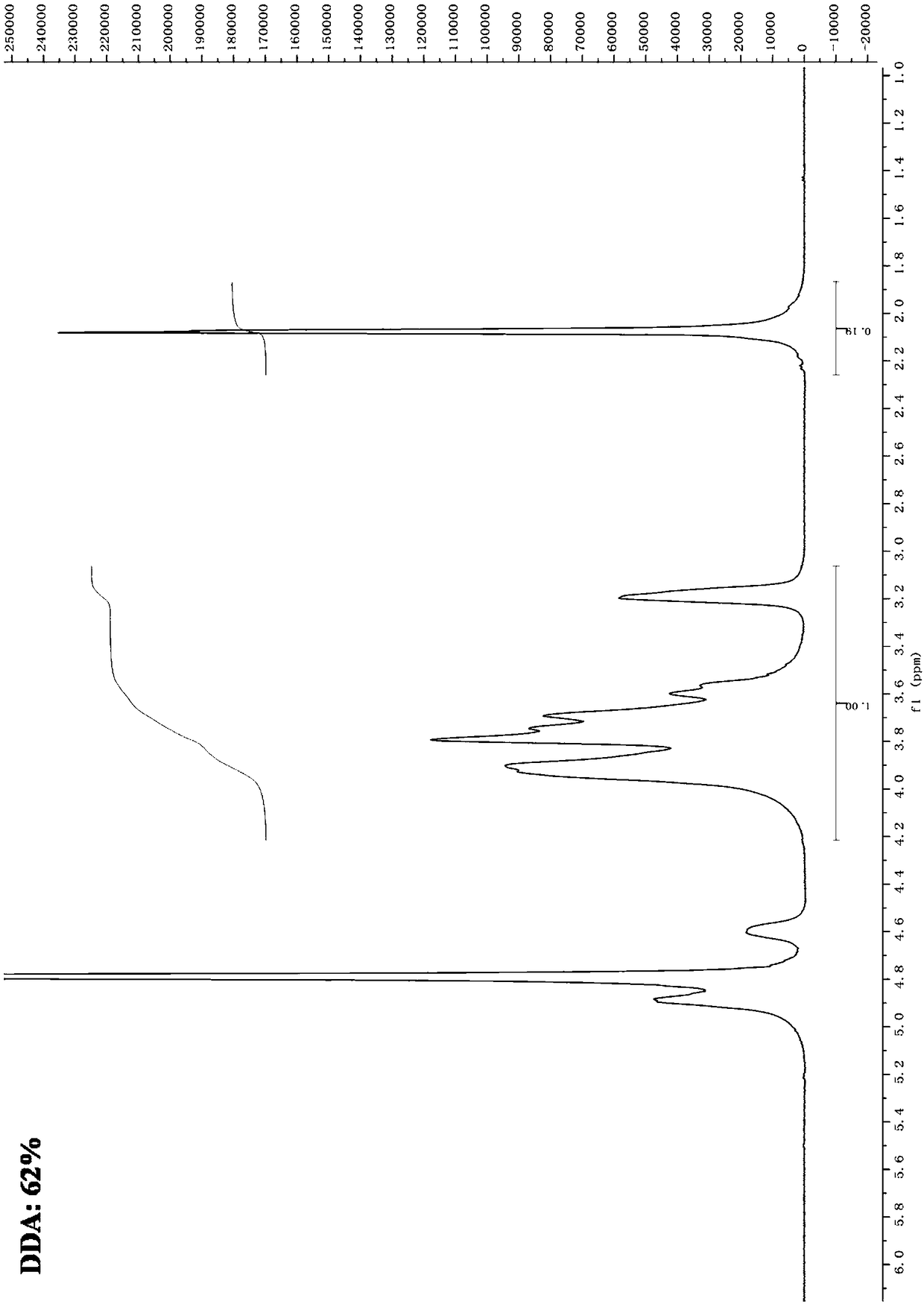
[0032] Embodiment 1: Preparation of chitosan oligosaccharides with low deacetylation degree of different structural types
[0033] Refer to the method of Liu Dasheng et al. Acetyl chitosan, the specific method is as follows: Weigh 30g of ultra-finely pulverized chitin powder, add it to 10 times of 45% sodium hydroxide solution, stir evenly, then heat up to 60°C for 2h deacetylation reaction, and react After centrifugation, the precipitate is washed with 60% ethanol-water mixed solution until it is free of alkalinity, and the product is obtained after drying. use 1 H-NMR measures its degree of deacetylation (see figure 1 ),according to figure 1 of 1 The H-NMR spectrum confirmed that the degree of deacetylation was 62%. Two portions of prepared chitosan were weighed, 10 g each, respectively added to a constant-temperature reaction kettle filled with 200 mL of 1.5% acetic acid aqueous solution, fully stirred to make it completely dissolved. Adjust the reaction temperature t...
Embodiment 2
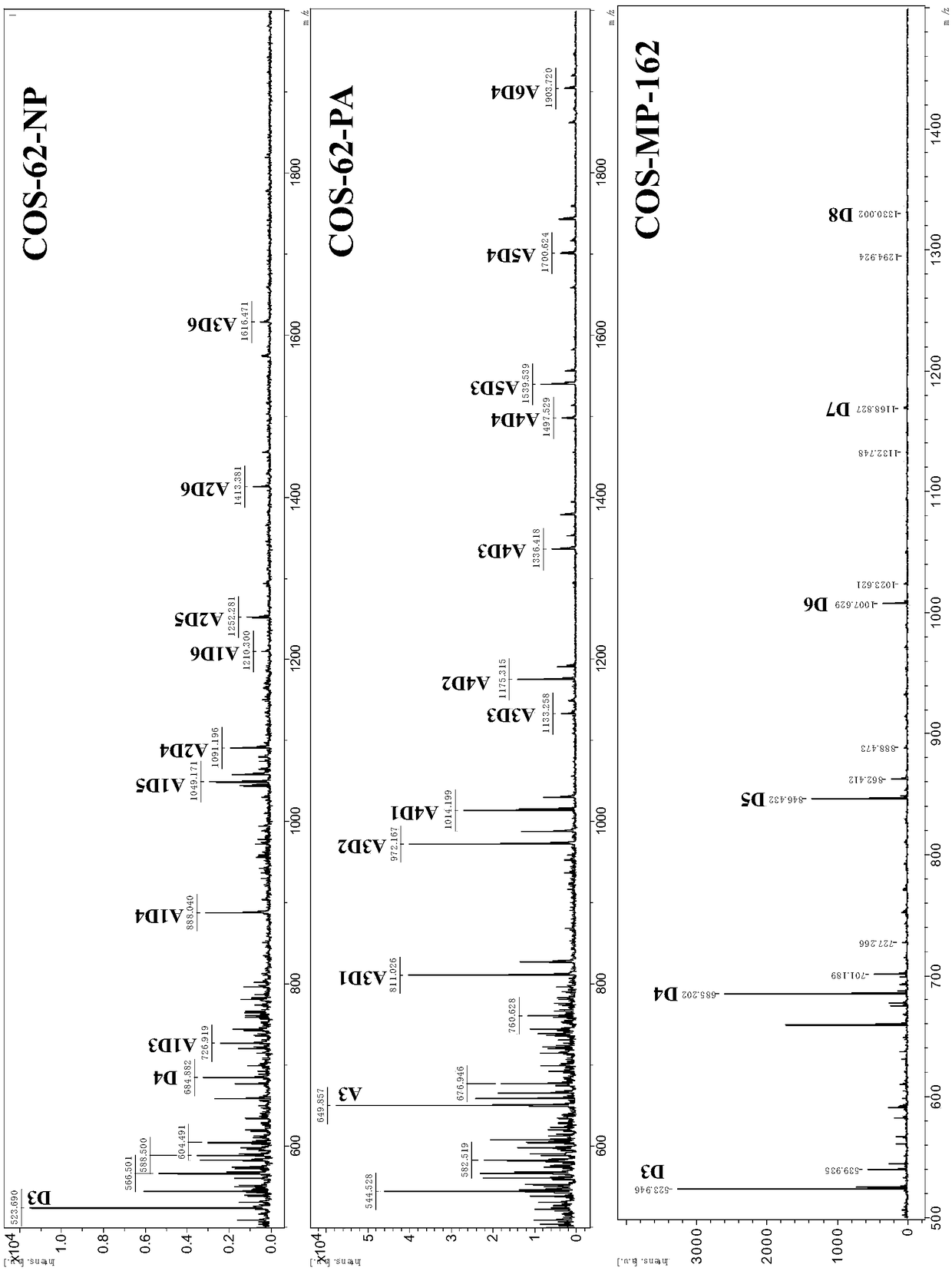
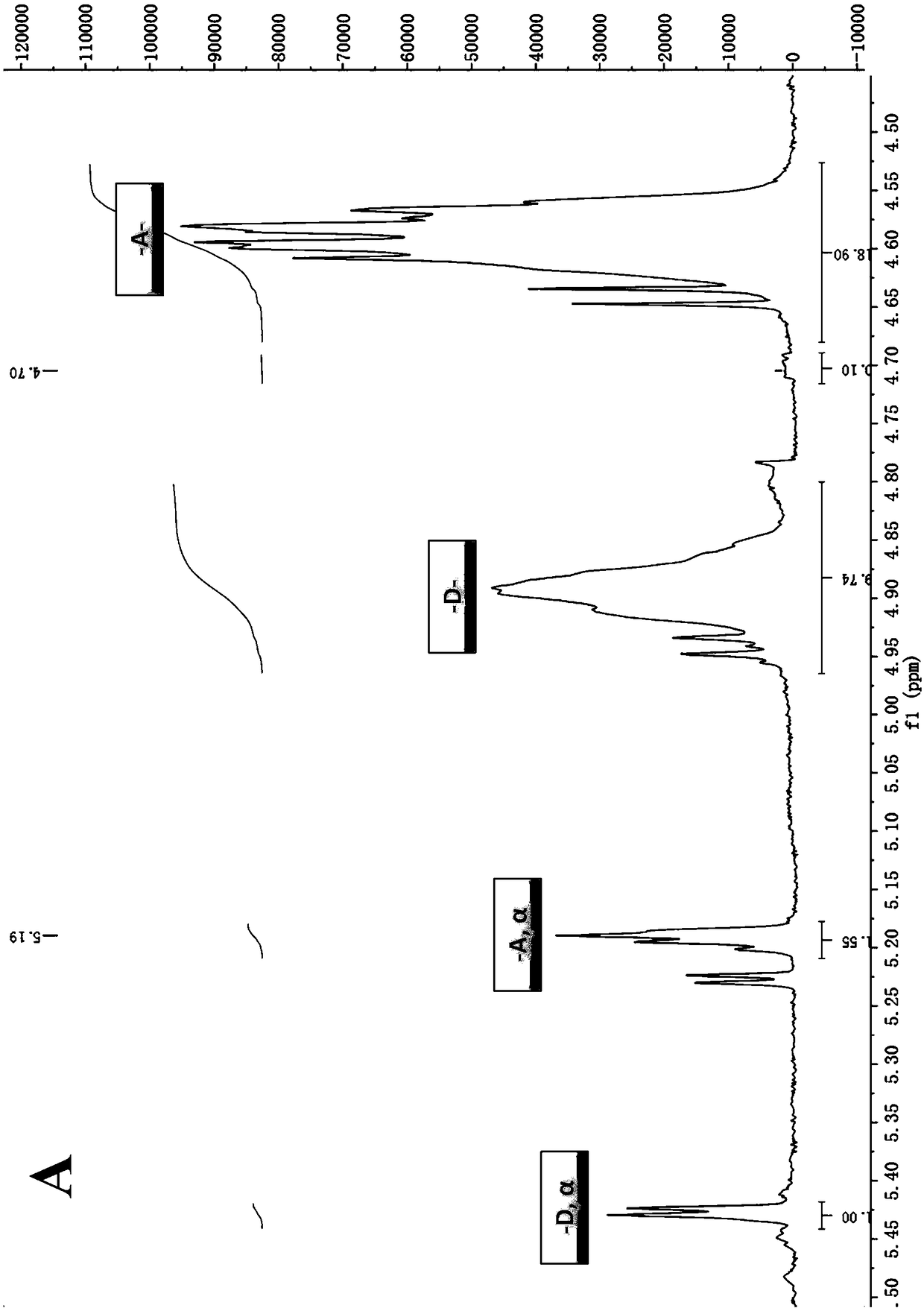
[0034] Embodiment 2: Composition and structural identification of different types of chitosan oligosaccharides
[0035] The chitosan oligosaccharide components in the above three chitosan oligosaccharides were identified by MALDI-TOF mass spectrometry. The specific method is: weigh three prepared or purchased chitosan oligosaccharide samples COS-62-NP, COS-62-PA and COS-MP-162, and prepare an aqueous solution with a concentration of 2 mg / mL with ultrapure water, and draw each 1 μL was applied to the sample plate, and after it was naturally dried, 1 μL of matrix 2,5-dihydroxybenzoic acid (DHB) solution was added, and after drying, the autoflexⅢ smartbeam MALDI-TOF mass spectrometer (Bruker Company) was used for detection ( positive ion reflectance mode). Mass spectrometry results such as figure 2 Shown: Mass spectra corresponding to COS-62-NP, COS-62-PA and COS-MP-162 respectively; for easy distinction, in figure 2 A stands for N-acetylglucosamine, D stands for glucosamine...
Embodiment 3
[0037] Embodiment 3: Comparison of anti-liver cancer activity of different structural types of chitosan oligosaccharides
[0038] Digest the human liver cancer cell line HepG2 cultured to the logarithmic phase and add MEM medium (10% FBS, S / P, 1% NAEE) to dilute to 1×10 4 Cells / ml, 100μl / well inoculated three 96-well cell culture plates, 37℃5%CO 2 Culture overnight in an incubator until the cells are completely attached. Three kinds of chitosan oligosaccharides (COS-62-NP, COS-62-PA and COS-MP-162) aqueous solutions (10 mg / ml) were prepared and sterilized by filtration with a 0.22 μm filter membrane in an intercellular ultra-clean bench. Dilute the three kinds of chitosan oligosaccharide aqueous solutions prepared above to 200 μg / ml with MEM medium (take 60 μl of 10 mg / ml oligosaccharide solution and add MEM medium 2940 μl to 3ml), 100 μl / well inoculates three 96-well cell culture plates ( Chitooligosaccharide final concentration is 100μg / ml), 37 ℃ 5% CO 2 Continue to grow ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| degree of polymerization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com