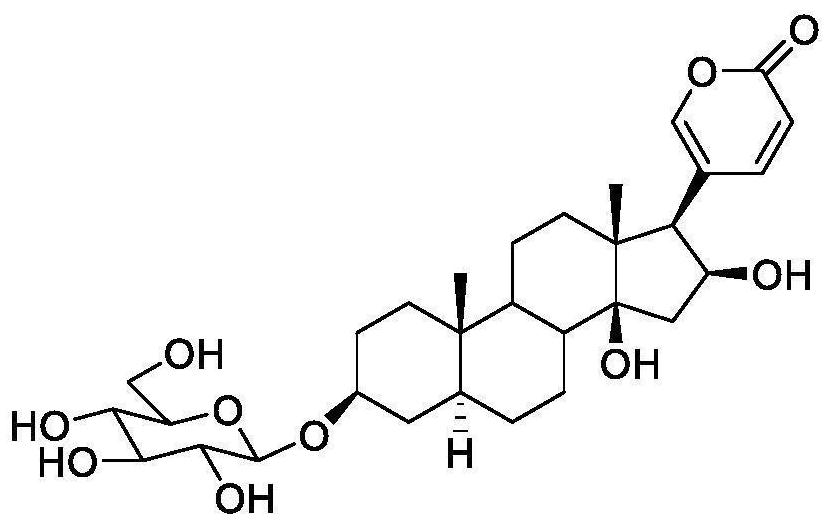
Preparation and anti-liver cancer application of 5alpha-structured type B cardiac glycoside
A cardiac glycoside and type B technology, applied in the field of natural medicinal chemistry, can solve the problems of poor prognosis, multi-drug resistance, and low immunity of the body, and achieve good anti-liver cancer activity, simple and convenient operation, and low cost Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0035] Example 1 Preparation of iron chopstick n-butanol extract
[0036] The collected iron chopsticks is naturally ventilated, weighted 7.5 kg. The crushing to a particle size diameter of less than 3 mm, soaked 4 times with 95% ethanol, 15 L each time, 7 days each time. The solvent was removed by a rotary evaporator to obtain a concentrated liquid 4L; EtOAc EtOAc (EtOAc) EtOAc (EtOAc) N-butanol was added, extracted four times, and the n-butanol phase was obtained, and the water phase was discarded. The n-butan alcohol extract was concentrated under reduced pressure with a rotary evaporator to give a brown red slurry as a bold product (650 g).
Embodiment 2
[0037] Example 2 Preparation of Strong Catosin
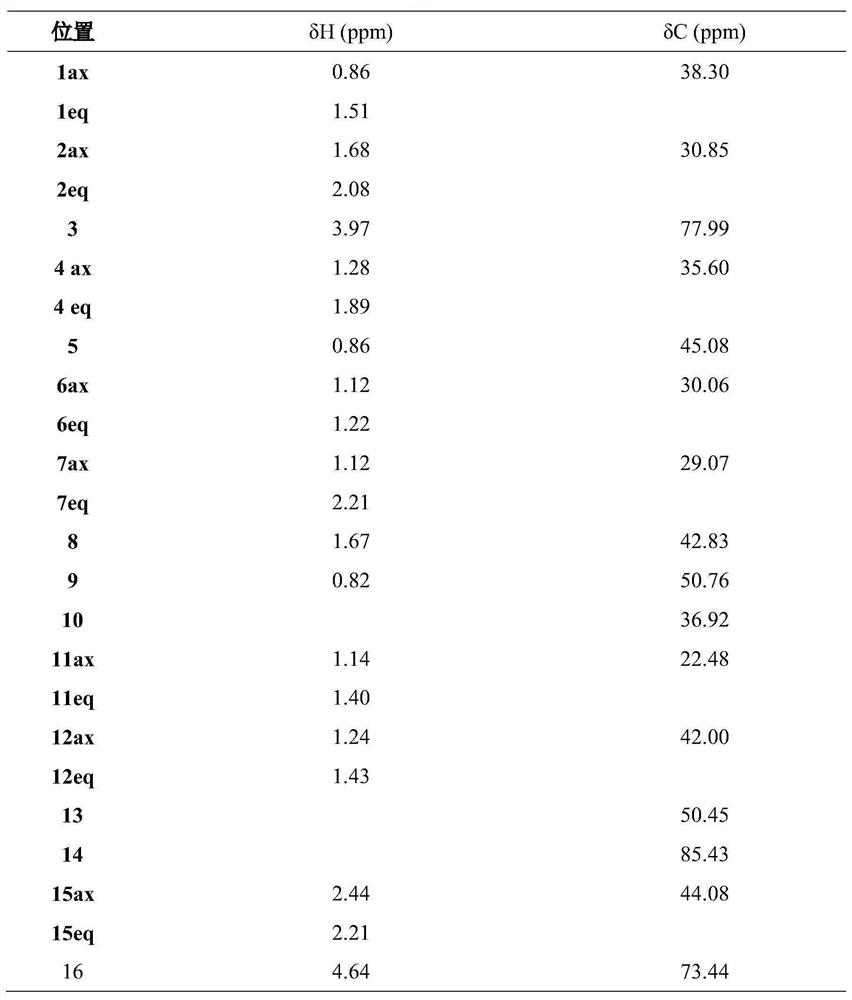
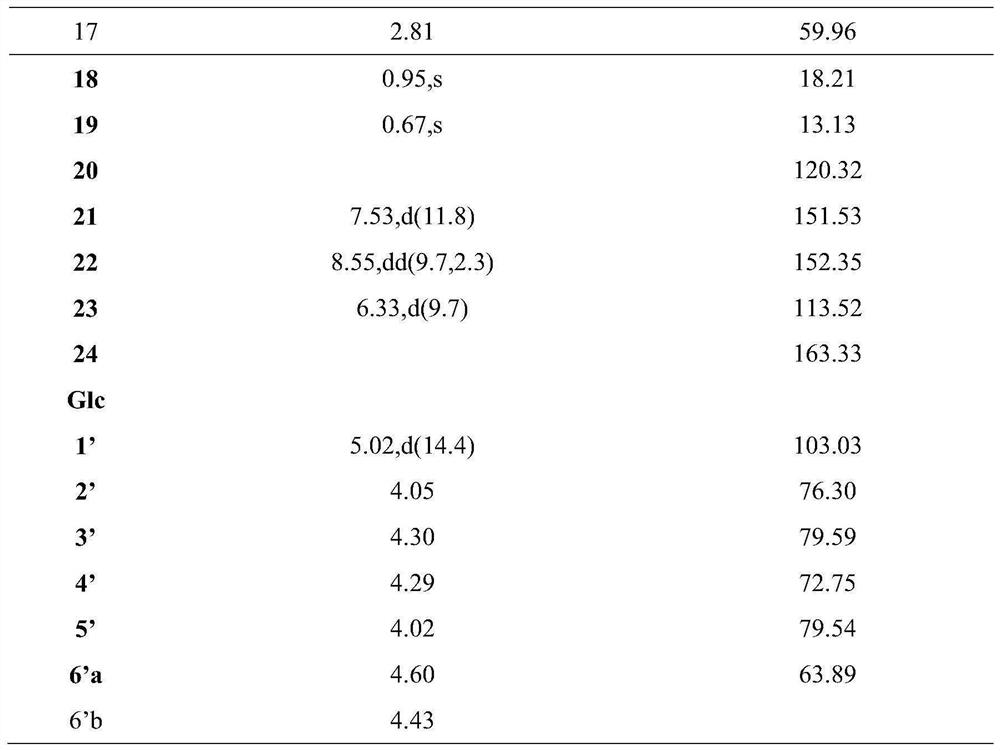
[0038] The iron chopsticks were concentrated to 500 mL, showing a deep brown brown-brown-brown viscous liquid, using D101 macroporous resin column chromatography, water, 30%, 50%, 95% ethanol gradient, and finally distilled concentrate Four fractions. The n-butanol 50% (65.8 g) was eluted with EtOAc Ethanol: Water (35%, 50%, 60%, 80%) gradient elution, four components FR.1-fr. 4. FR.1 first elution with n-phase silica gel chromatography eluting with dichloromethane: methanol (15: 1, 8: 1, 5: 1, and 2: 1) gradient, five components FR.1.1-fr. 1.5. FR.1.2 (800 mg) was eluted with methanol-washing release gradient in the reversed chromatography column, and five components were obtained by FR.1.2.1-Fr.1.2.5. FR.1.2.3 is first in the gel column (dichloromethane: methanol = 1: 1), silica gel column (dichloromethane: methanol = 13: 1, 8: 1, 5: 1) further repeatedly purified, and finally The preparative high performance liquid phase (the chr...
Embodiment 3
[0041] Example 3 Strong Chanosine compound anti-liver cancer activity
[0042] Hepatocellular HepG2, rat liver star cells HSC-T6 were added at 37 ° C at 37 ° C in RPMI-1640 medium added 2.05 mm / l glutamine and 10% FBS, at 5% CO 2 Culture in humidification environments. Cells were isolated from the culture flask from the culture flask using trypsin EDTA. Dilute to 5 × 10 with a complete medium 4 After / ml, 100 μl of the cell suspension was added to each of the 96-well cultural sheets. It was then cultured in a 5% carbon dioxide environment at 37 ° C. The test compound of the specified concentration was added to each well and then cultured for 48 h. Dooricin as a positive control. The attached cells were fixed with cold 50% trichloroacetic acid for 30 min and then stained with 0.04% SrB for 30 min. After dissolving the bound SRB in a Tris medium of 10 mM, the absorbance at 450 nm was measured with a TECAN multifunctionramid instrument.
[0043] Anti-liver cancer activity such as ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com