A method of continuously synthesizing hexanedioic acid through a microchannel reactor
A technology of microchannel reactor and adipic acid, which is applied in the field of continuous reaction technology, can solve the problems of high system pressure, low yield of adipic acid, gasification of cyclohexene, etc., to maintain the reaction temperature, increase the reaction rate, The effect of enhancing mass transfer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
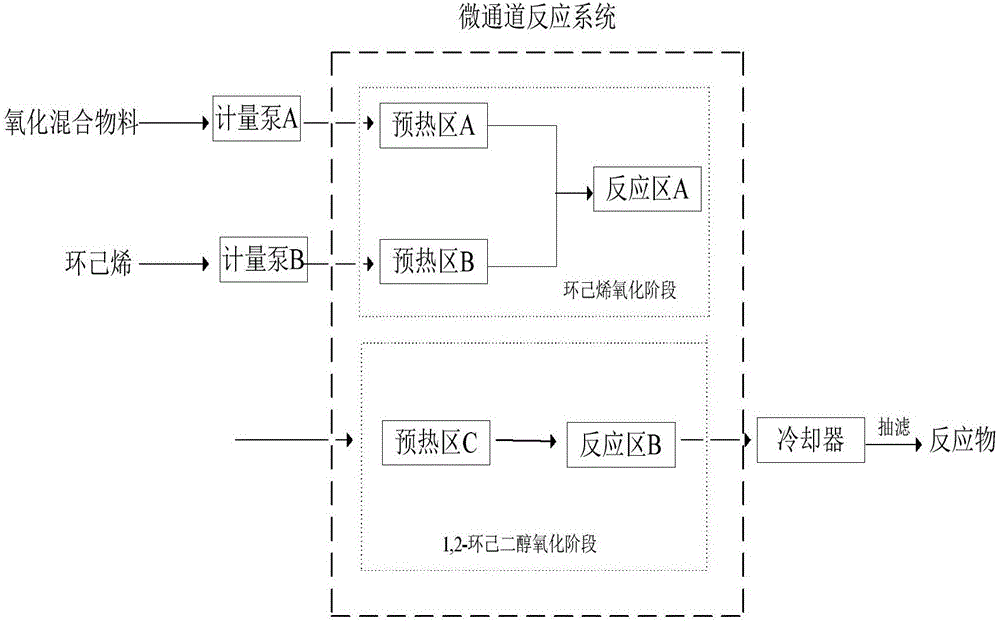
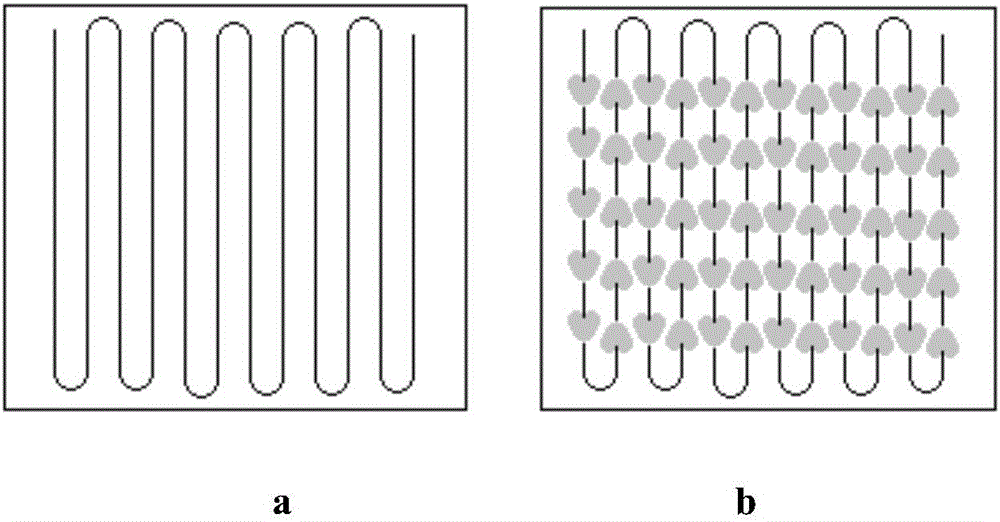
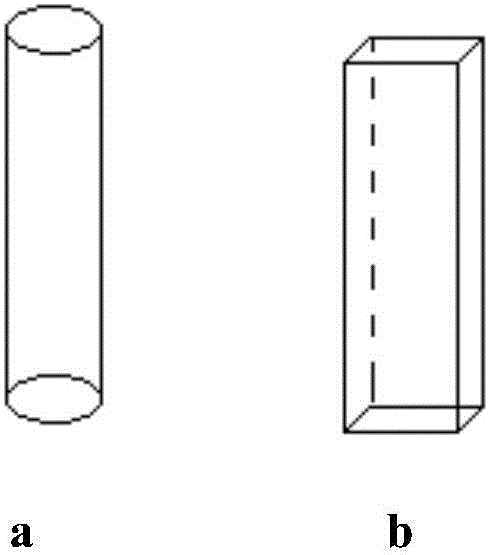
[0038] (1) Microchannel reaction system: Preheating zones A, B, and C adopt a circular structure, and reaction zones A and B adopt a heart-shaped mixing structure. The inner diameter of the flow channel is 6mm, and the size of the stirring blade is 6mm×1mm.
[0039] (2) Cyclohexene oxidation stage: the number of preheating modules in preheating zone A and B is 2, the number of reactor modules in reaction zone A is 16, and the total reaction volume is 128mL. Choose tungstic acid as catalyst, w(H 2 o 2 )=10%, according to the material molar ratio: n(H 2 o 2 ): n (cyclohexene): n (tungstic acid) = 4.9:1:0.01 Prepare the reaction materials, control the flow rate of the materials, and the residence time is 30min. The temperature of the hexene oxidation stage is 90°C, and the two streams of materials are mixed and reacted in the reaction zone A, and the effluent is analyzed, and the conversion rate of cyclohexene is 100%.
[0040] (3) 1,2-cyclohexanediol oxidation stage: the num...
Embodiment 2
[0042] (1) Microchannel reaction system: the preheating zones A, B, and C adopt a rectangular structure, and the reaction zones A and B adopt a triangular mixing structure. The inner diameter of the flow channel is 3mm, and the size of the stirring blade is 4mm×0.6mm.
[0043] (2) Cyclohexene oxidation stage: the number of preheating modules in preheating zone A and B is 2, the number of reactor modules in reaction zone A is 10, and the total reaction volume is 80mL. Choose sodium tungstate as catalyst, w(H 2 o 2 )=70%, according to the material molar ratio: n(H 2 o 2 ):n(cyclohexene):n(sodium tungstate)=6:1:0.12 Prepare the reaction materials, control the flow rate of the materials, and the residence time is 10min. The temperature in the cyclohexene oxidation stage is 50°C, and the two streams of materials are mixed and reacted in the reaction zone A, and the effluent is analyzed, and the conversion rate of cyclohexene is 100%.
[0044] (3) 1,2-cyclohexanediol oxidation s...
Embodiment 3
[0046] (1) Microchannel reaction system: the preheating zones A, B, and C adopt a rectangular structure, and the reaction zones A and B adopt a circular mixing structure. The inner diameter of the flow channel is 0.5mm, and the size of the stirring blade is 0.8mm×0.3mm.
[0047] (2) Cyclohexene oxidation stage: the number of preheating modules in preheating zone A and B is 1, the number of reactor modules in reaction zone A is 2, and the total reaction volume is 16mL. Choose tungstic acid as catalyst, w(H 2 o 2 )=70%, according to the material molar ratio: n(H 2 o 2 ):n(cyclohexene):n(tungstic acid)=5.5:1:0.12 Prepare the reaction materials, control the flow rate of the materials, and the residence time is 1min. The temperature of the hexene oxidation stage is 70°C, and the two streams of materials are mixed and reacted in the reaction zone A, and the effluent is analyzed, and the conversion rate of cyclohexene is 100%.
[0048] (3) 1,2-cyclohexanediol oxidation stage: the...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com