Preparation method of mono-/multi-metal coprecipitation hydroxide or carbonate
A hydroxide and carbonate technology, applied in the preparation of carbonate/acid carbonate, oxygen/ozone/oxide/hydroxide, nickel oxide/nickel hydroxide, etc., can solve pipeline blockage , unsatisfactory, low solubility and other problems, to achieve the effect of solving pipeline blockage, improving tap density and good sphericity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
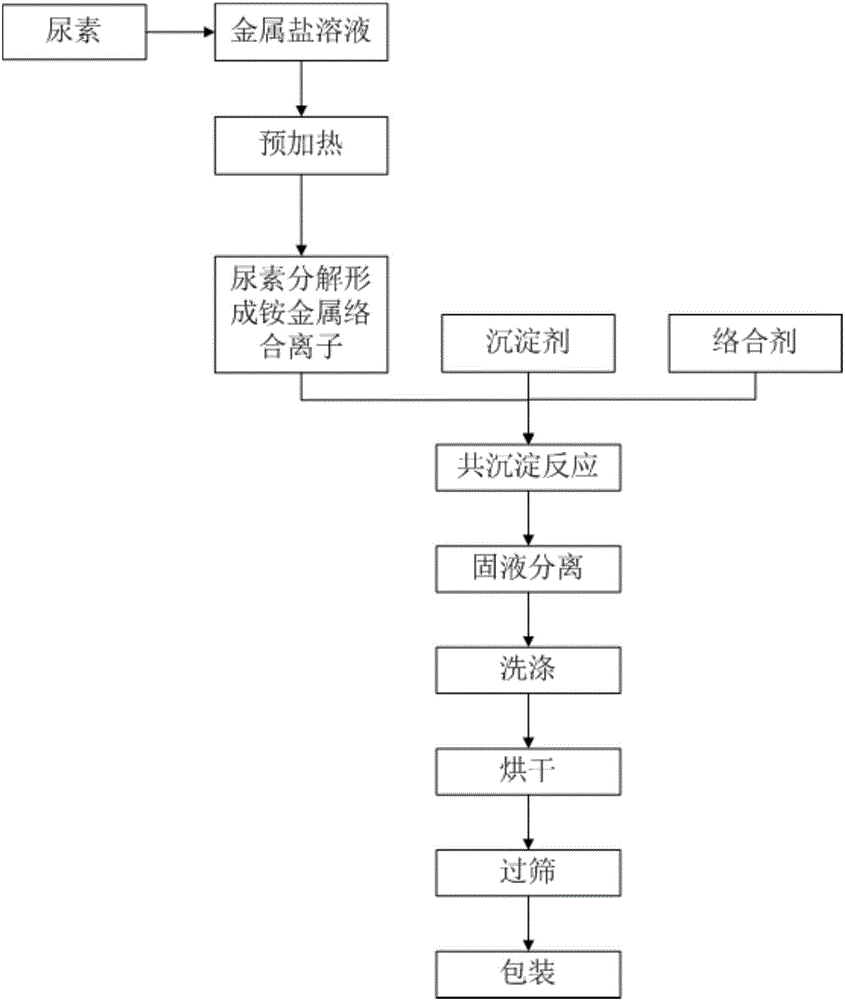
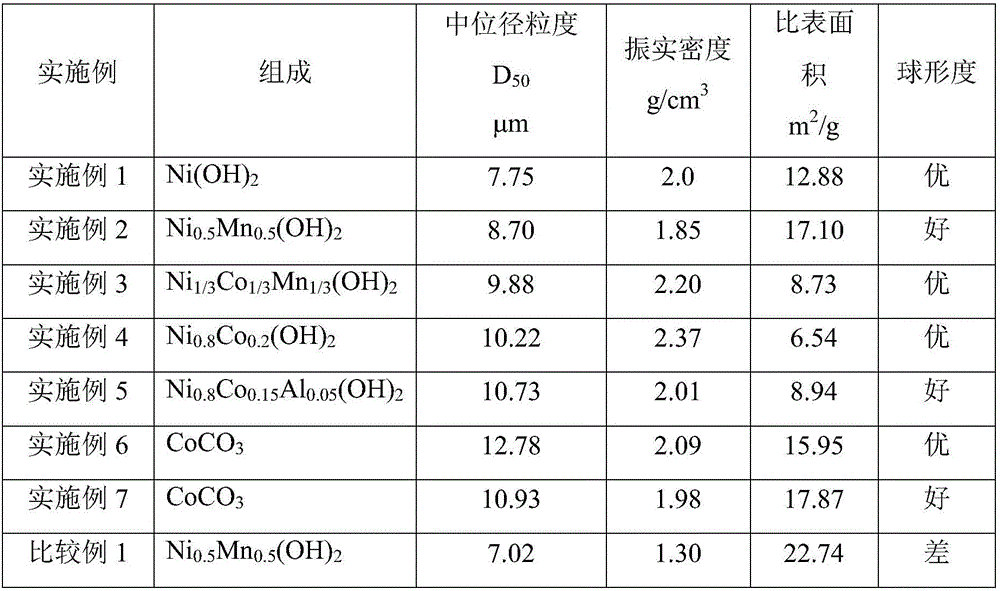
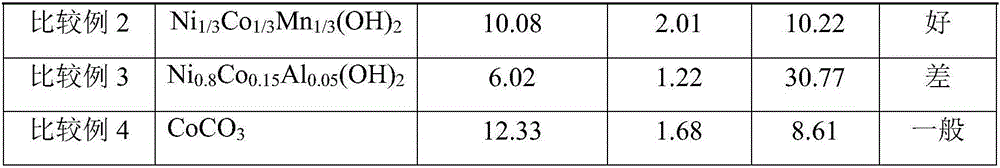
[0032] In the nickel sulfate solution that concentration is 0.1mol / L, add urea in the ratio of 3g / L, at this moment, urea and metal Ni in the solution 2+ The substance mass ratio is 0.5:1. The precipitation agent is sodium hydroxide with a concentration of 6mol / L, and the complexing agent is ammonia water with a concentration of 20wt%. Add deionized water to the reactor in advance until the bottom paddle is submerged, then add ammonia water to adjust the pH value to 10.5-11.0, and then raise the temperature in the reactor to 60-70°C. With the silicone rubber tube with an inner diameter of 10mm as the delivery pipeline, the nickel sulfate solution is pumped into the reaction kettle at a speed of 5ml / min, and the length of the pipeline entering the water bath is adjusted to be 6.5cm. The residence time of the nickel sulfate solution in the water bath is The time is 1min. Ammonia is pumped into the reaction kettle simultaneously at a speed of 2.8ml / min (ammonia is divided into ...
Embodiment 2
[0034] In the nickel-manganese sulfate solution that concentration is 1mol / L, add urea by the ratio of every liter 6g, Ni / Mn=1 in the solution, at this moment, the amount of urea in the solution is equal to that of metal Ni 2+ with Mn 2+ The total mass ratio of the substance is 2:1. The precipitation agent is sodium hydroxide with a concentration of 4mol / L, and the complexing agent is ammonia water with a concentration of 20wt%. Add deionized water to the reactor in advance until the bottom paddle is submerged, then add ammonia water to adjust the pH value to 10.5-11.0, and then raise the temperature in the reactor to 60-70°C. With a silicone rubber tube with an inner diameter of 10mm as the delivery pipeline, the salt solution is pumped into the reaction kettle at a speed of 50ml / min, and the length of the pipeline entering the water bath is adjusted to 200cm, and the residence time of the salt solution in the water bath is 3.1 min. Ammonia water is pumped into the reactio...
Embodiment 3
[0036] In the nickel-cobalt-manganese sulfate solution that concentration is 2mol / L, add urea in the ratio of every liter 360g, Ni / Co / Mn=1 in the solution, now in the solution, the amount of urea and metal (Ni 2+ +Co 2+ +Mn 2+ ) The total substance mass ratio is 2:1. The precipitation agent is sodium hydroxide with a concentration of 10mol / L, and the complexing agent is ammonia water with a concentration of 20wt%. Add deionized water to the reactor in advance until the bottom paddle is covered, then add ammonia water to adjust the pH value to 10.5-11.0, and then raise the temperature in the reactor to 50-60°C. Use a silicone rubber tube with an inner diameter of 10mm as the delivery pipeline, pump the salt solution into the reaction kettle at a speed of 100ml / min, adjust the length of the pipeline entering the water bath to 1275cm, and the residence time of the salt solution in the water bath is 10min . Ammonia water is pumped into the reaction kettle simultaneously with t...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com