Method for preparing vanadyl sulfate by use of vanadium-containing chloride solution
A technology of vanadyl sulfate and vanadyl sulfate solution, applied in the preparation of vanadium compounds, vanadium compounds, chemical instruments and methods, etc., can solve problems such as high cost, influence product purity, etc., and achieve high recovery rate, high product quality, Avoid energy-intensive effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
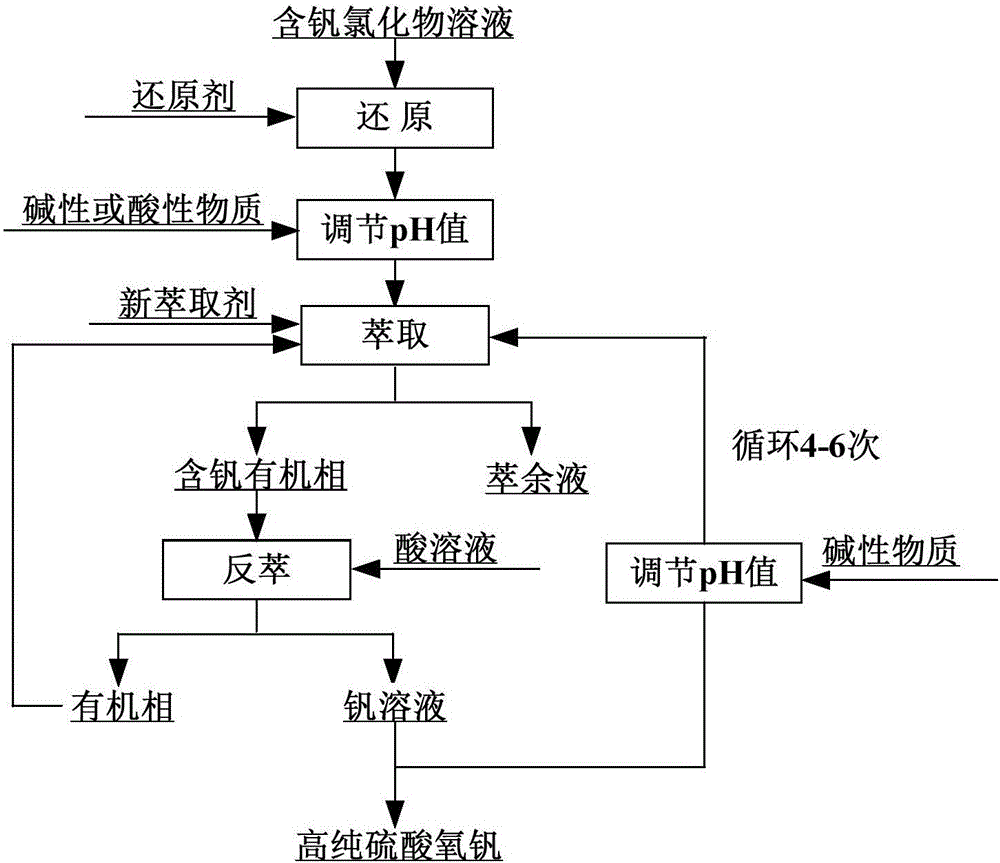
[0034] The main components of the vanadium-containing chloride solution are: the V content is 8.3g / L, the Fe content is 78.7g / L, the Ca content is 17.4g / L, the Mg content is 1.1g / L, and the Al content is 9.4g / L;
[0035] 1) Heating the solution to 30°C, stirring, adding iron powder for reduction, reducing V(Ⅴ) in the solution to V(Ⅳ), reducing Fe(Ⅲ) to Fe(II), and filtering to obtain a reduced solution;
[0036] 2) Step 1) is used to obtain the reduction solution using Na 2 CO 3 Adjust the pH value of the reducing solution to 0.5, and filter;
[0037] 3) The solution obtained in step 2) is mixed with a mixed extractant of P507, TBP and kerosene (volume ratios of 5%, 5%, and 90% respectively) for extraction, O / A=2:1, and the vanadium-loaded organic phase is obtained;
[0038] 4) Back-extract the vanadium-loaded organic phase obtained in step 3) with 10% sulfuric acid solution, O / A=1:1, to obtain a vanadium solution;
[0039] 5) the vanadium solution obtained in step 4) is ad...
Embodiment 2
[0044] The main components of the vanadium-containing chloride solution are: the V content is 15.3g / L, the Fe content is 14.7g / L, the Ca content is 5.3g / L, the Mg content is 4.1g / L, and the Al content is 3.4g / L;
[0045] 1) Heating the solution to 90°C, stirring, adding sodium sulfite for reduction, reducing V(Ⅴ) in the solution to V(Ⅳ), reducing Fe(Ⅲ) to Fe(II), and filtering to obtain a reduced solution;
[0046] 2) Using hydrochloric acid to adjust the pH value of the reducing solution to 1.1 in the reducing solution obtained in step 1);
[0047] 3) Mix the filtered solution obtained in step 2) with a mixed extractant of P507, TBP and n-heptane (volume ratios of 25%, 15%, and 60% respectively) for extraction, O / A=1:2, and obtain loaded vanadium The organic phase;
[0048]4) Back-extract the vanadium-loaded organic phase obtained in step 3) with 20% sulfuric acid solution, O / A=1:1, to obtain a vanadium solution;
[0049] 5) the vanadium solution obtained in step 4) is adju...
Embodiment 3
[0054] The main components of the vanadium-containing chloride solution are: the V content is 3.5g / L, the Fe content is 0.3g / L, the Ca content is 3.1g / L, and the Al content is 2.7g / L;
[0055] 1) Heating the vanadium-containing chloride solution to 60° C., stirring, adding sodium sulfite for reduction, reducing V(Ⅴ) in the solution to V(Ⅳ) and Fe(Ⅲ) to Fe(II), and filtering to obtain a reduced solution;
[0056] 2) Using calcium carbonate to adjust the pH value of the reducing solution to 2 in the reducing solution obtained in step 1);
[0057] 3) The filtered solution obtained in step 2) is mixed with a mixed extractant of P204, TBP and n-heptane (volume ratios of 40%, 5%, and 55% respectively) for extraction, O / A=1:4, and the loaded vanadium is obtained The organic phase;
[0058] 4) Back-extract the vanadium-loaded organic phase obtained in step 3) with 40% sulfuric acid solution, O / A=1:1, to obtain a vanadium solution;
[0059] 5) the vanadium solution obtained in step 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com