Superparamagnetic Fe3O4 coated by oleic acid and preparation method thereof
A superparamagnetic, fe3o4 technology, applied in the fields of nanotechnology, nanotechnology, nanotechnology, etc. for materials and surface science, it can solve the problems that nanoparticles are not easy to wash, require high process equipment, and harsh reaction conditions. Uniform size, high purity and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0037] (1) In the air, 7g FeCl 2 4H 2 O was added to 50 mL of deionized water to obtain a concentration of 0.14 g / mL of FeCl 2 aqueous solution. to 50mL FeCl 2 Add 30 mL of ammonia water to the aqueous solution, and after stirring for 20 minutes, the color gradually turns light green, then dark green, and finally black;
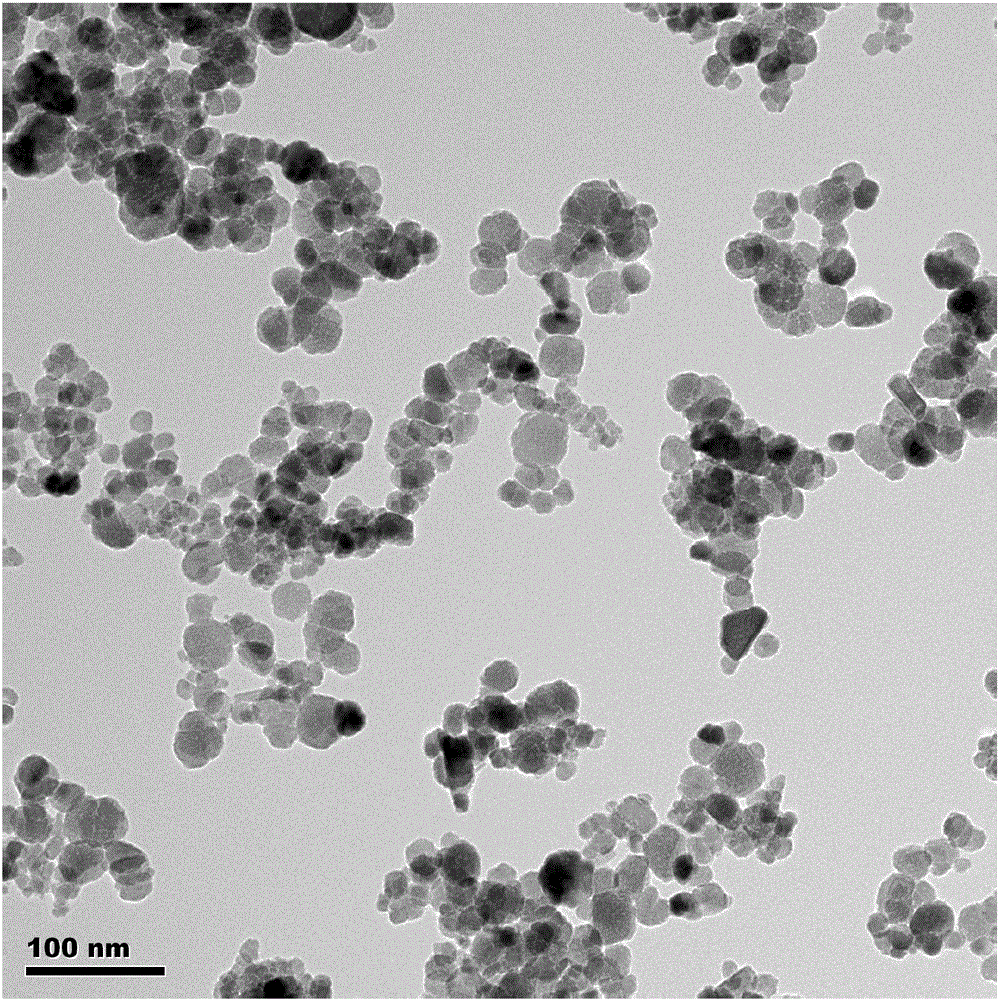
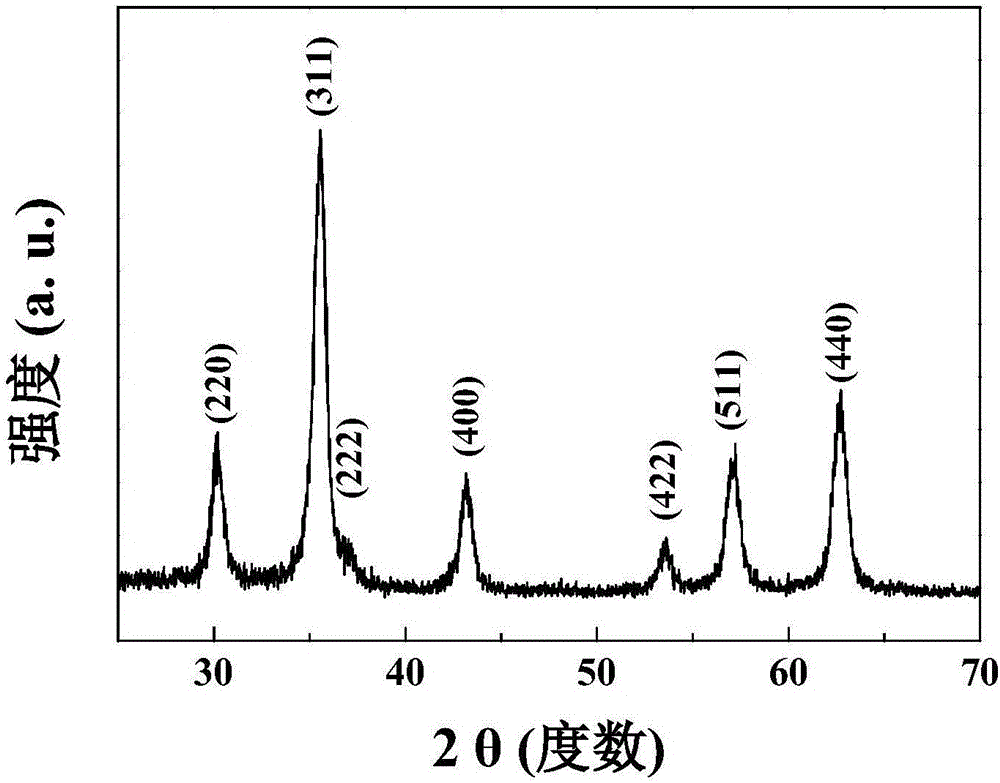
[0038] (2) Add 1.1 g of oleic acid to step (1), mix evenly, place the mixed solution in a closed reaction kettle, heat and react at 110°C for 4 hours, then alternately wash twice with deionized water and ethanol , after magnetic separation, disperse in n-hexane to obtain the black superparamagnetic nano Fe coated with oleic acid 3 o 4 1. The particle size is 12.0±3.26nm, and the average particle size is 12nm.
Embodiment 2
[0040] (1) In the air, 10g FeCl 2 4H 2 O was added to 100 mL of deionized water to obtain a concentration of 0.10 g / mL of FeCl 2 aqueous solution. to 100mL FeCl 2 Add 10 mL of ammonia water to the aqueous solution, and after stirring for 60 minutes, the color gradually turns light green, then dark green, and finally black;
[0041] (2) Add 1 g of oleic acid to step (1), mix evenly, place the mixed solution in a closed reaction kettle, heat and react at 60° C. for 5 hours, then alternately wash twice with deionized water and ethanol, After magnetic separation, disperse in octyl hexane to obtain the black superparamagnetic nano Fe coated with oleic acid 3 o 4 2. The average particle size is 8nm.
Embodiment 3
[0043] (1) In the air, 50g FeCl 2 4H 2 O was added to 100 mL of deionized water to obtain a concentration of 0.50 g / mL of FeCl 2 aqueous solution. to 100mLFeCl 2 Add 50mL of ammonia water to the aqueous solution, and after stirring for 90 minutes, the color gradually turns light green, then dark green, and finally black;
[0044] (2) Add 5g of oleic acid to step (1), after mixing evenly, place the mixed solution in a closed reaction kettle, heat and react at 90°C for 5 hours, then alternately wash each three times with deionized water and ethanol, and magnetically After separation, disperse in n-octane to obtain the black superparamagnetic nano Fe coated with oleic acid 3 o 4 3. The average particle size is 20nm.
PUM
| Property | Measurement | Unit |
|---|---|---|
| The average particle size | aaaaa | aaaaa |
| The average particle size | aaaaa | aaaaa |
| Saturation magnetization | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com