Purifying method for 1,2-epoxybutane
A technology of butylene oxide and purification method, which is applied in the direction of organic chemistry, can solve the problems of low yield and large loss of extractant, and achieve the effect of high separation efficiency and low equipment investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0044] The crude 1,2-epoxybutane solution is calculated by mass percentage, 93.5% of 1,2-epoxybutane, 5% of water, and the rest are impurities of propionaldehyde, methanol and methyl formate.
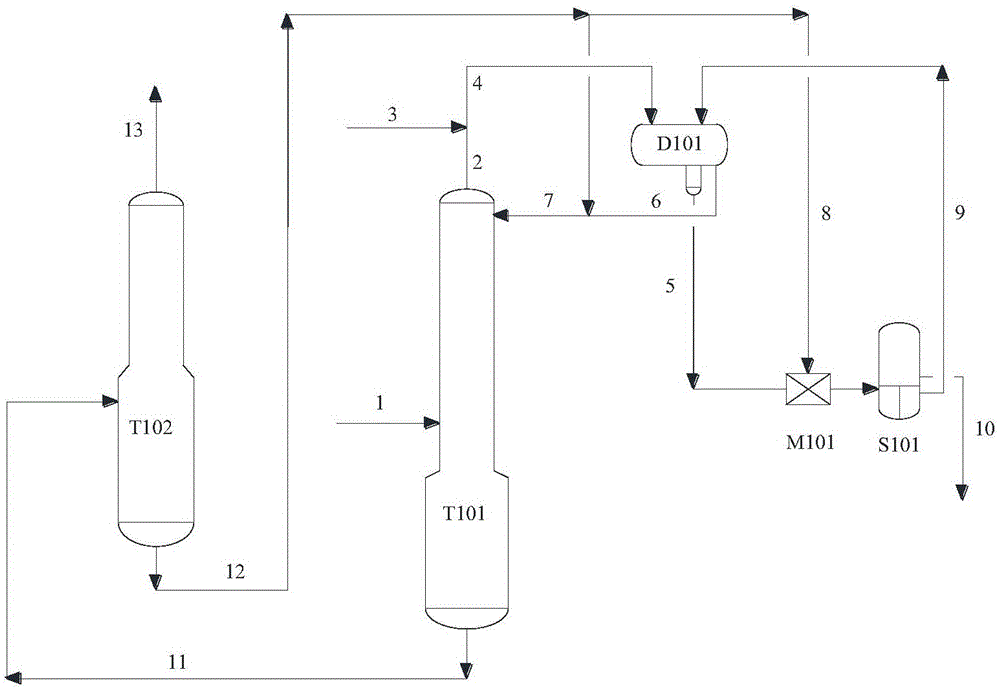
[0045] Using the extractive distillation column T101, the crude 1,2-epoxybutane solution 1 containing impurities is supplied to C 7 ~C 12 In the extractive distillation where hydrocarbons are used as the extractant; medium-pressure steam or deionized water 3 is sprayed into the overhead gas 2 of the extractive distillation tower T101, and the condensate 4 is separated into an oil layer 6 and a water layer 5 in the phase separator D101, The oil layer 6 and the recovery extractant 12 are circulated to the extractive distillation tower T101 as the extractant and also as reflux 7; the liquid 11 of the extractive distillation tower T101 enters the solvent recovery tower T102, and after separation, 1,2-epoxybutane is obtained at the top of the tower Product 13, solvent recovery tower liquid ...
Embodiment 2
[0054] The crude 1,2-epoxybutane solution is expressed in mass percent, 94.5% of 1,2-epoxybutane, 4% of water, and the rest are impurities of propionaldehyde, methanol and methyl formate.
[0055] The implementation mode is the same as [Example 1], the difference is:
[0056] The theoretical plate number of the extractive distillation column is 45, the operating pressure is 160kPa, and the operating temperature is 78°C.
[0057] The solvent ratio of the extractive distillation column was 5.
[0058] The theoretical plate number of the solvent recovery tower is 35, the operating pressure is 160kPa, and the operating temperature is 80°C.
[0059] The operating pressure of the liquid-liquid separator is 130kPa, and the operating temperature is 40°C.
[0060] Extractant A is isooctane.
[0061] The weight ratio of stream I to stream II is 600:1.
[0062] In terms of mass percentage, the purity of 1,2-butylene oxide in stream 13 reaches 99.96%, propionaldehyde is 10ppm, and n-o...
Embodiment 3
[0064] The crude 1,2-epoxybutane solution is expressed in mass percent, 96% of 1,2-epoxybutane, 2% of water, and the rest are impurities of propionaldehyde, methanol and methyl formate.
[0065] The implementation mode is the same as [Example 1], the difference is:
[0066] The theoretical plate number of the extractive distillation tower is 60, the operating pressure is 200kPa, and the operating temperature is 85°C.
[0067] The solvent ratio of the extractive distillation column is 4.
[0068] The theoretical plate number of the solvent recovery tower is 40, the operating pressure is 200kPa, and the operating temperature is 90°C.
[0069] The operating pressure of the liquid-liquid separator is 160kPa, and the operating temperature is 45°C.
[0070] Extractant A is 2-methylpentane.
[0071] The weight ratio of stream I to stream II is 650:1.
[0072] In terms of mass percentage, the 1,2-epoxybutane product in stream 13 has a purity of 99.98%, 5 ppm of propionaldehyde, an...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com