Kangshen granule fingerprint construction method and standard fingerprint thereof
A standard fingerprint and construction method technology, applied in the construction method of Kangshen granule fingerprint and its standard fingerprint field, can solve the problems of shortage, simple quality standard, inability to control the quality of Kangshen granule, etc., and achieve good stability, The effect of effective quality control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
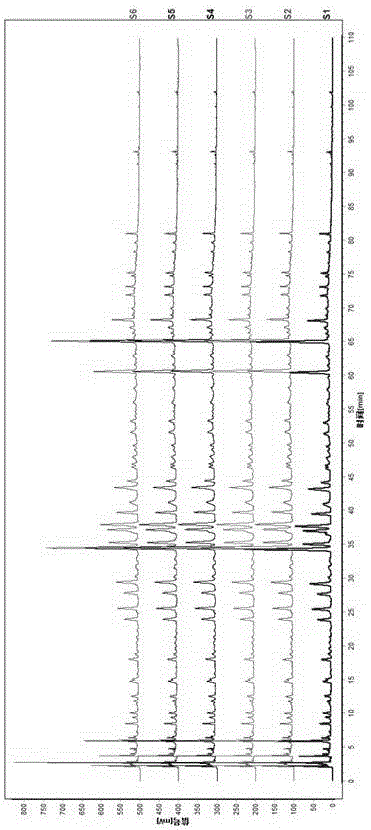
[0054] 1. Preparation of the test solution: take about 2.0 g of the sample, accurately weigh it, put it in a stoppered conical flask, add 25 ml of 75% methanol accurately, weigh it, extract it by ultrasonic extraction for 30 minutes, let it cool, and weigh it again. Determine the weight, make up the lost weight with 75% methanol, shake well, filter, and take the filtrate to obtain the final product.
[0055] 2. Preparation of reference substance solution: take puerarin, caffeic acid, daidzin, rosmarinic acid, hesperidin, daidzein reference substance, accurately weighed, add methanol to prepare a concentration of puerarin 90.0 μg / ml, A mixed reference solution of caffeic acid 52.6 μg / ml, daidzin 127.2 μg / ml, rosmarinic acid 80.4 μg / ml, hesperidin 19.1 μg / ml, daidzein 42.8 μg / ml.
[0056] 3. High performance liquid chromatography conditions: the chromatographic column uses octadecylsilane bonded silica gel as filler; the detection wavelength is 280nm; the column temperature is 2...
Embodiment 2
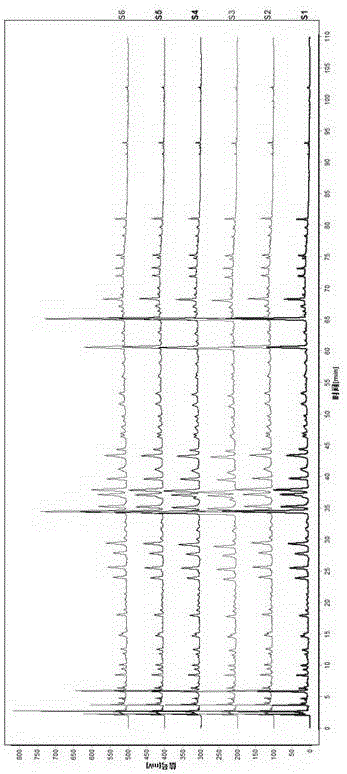
[0065] 1. Preparation of the test solution: take about 0.5g of this product, accurately weigh it, put it in a stoppered conical flask, add 15ml of 50% methanol accurately, weigh it, extract it by ultrasonic extraction for 15 minutes, let it cool, and then Weigh the weight, make up the lost weight with 50% methanol, shake well, filter, and take the filtrate to obtain the final product.
[0066] 2. Preparation of reference substance solution: take puerarin, caffeic acid, daidzin, rosmarinic acid, hesperidin, daidzein reference substance, accurately weighed, add methanol to prepare a concentration of puerarin 90.0 μg / ml, A mixed reference solution of caffeic acid 52.6 μg / ml, daidzin 127.2 μg / ml, rosmarinic acid 80.4 μg / ml, hesperidin 19.1 μg / ml, daidzein 42.8 μg / ml.
[0067] 3. High performance liquid chromatography conditions: the chromatographic column uses octadecylsilane bonded silica gel as filler; the detection wavelength is 270nm; the column temperature is 20°C; acetonitri...
Embodiment 3
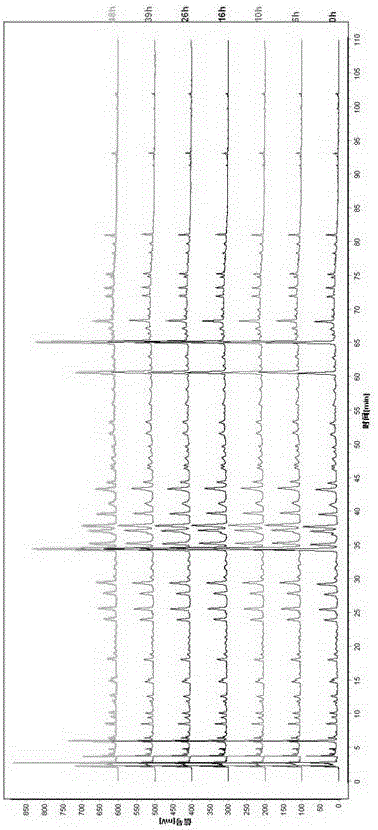
[0076] 1. Preparation of the test solution: take about 5.0 g of this product, accurately weigh it, put it in a stoppered conical flask, add 50 ml of 100% methanol accurately, weigh it, extract by heating and reflux for 60 minutes, let it cool, and then Weigh the weight, make up the lost weight with 100% methanol, shake well, filter, and take the filtrate to obtain the final product.
[0077] 2. Preparation of reference substance solution: take puerarin, caffeic acid, daidzin, rosmarinic acid, hesperidin, daidzein reference substance, accurately weighed, add methanol to prepare a concentration of puerarin 90.0 μg / ml, A mixed reference solution of caffeic acid 52.6 μg / ml, daidzin 127.2 μg / ml, rosmarinic acid 80.4 μg / ml, hesperidin 19.1 μg / ml, daidzein 42.8 μg / ml.
[0078] 3. High performance liquid chromatography conditions: the chromatographic column uses octadecylsilane bonded silica gel as filler; the detection wavelength is 290nm; the column temperature is 40°C; acetonitrile...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
| Wavelength | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com