Ordered catalyst layer and preparation and application thereof
A catalytic layer, catalyst technology, applied in nanotechnology, electrical components, battery electrodes, etc. for materials and surface science, to achieve the effect of improving utilization, reducing usage, and shortening conduction paths
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
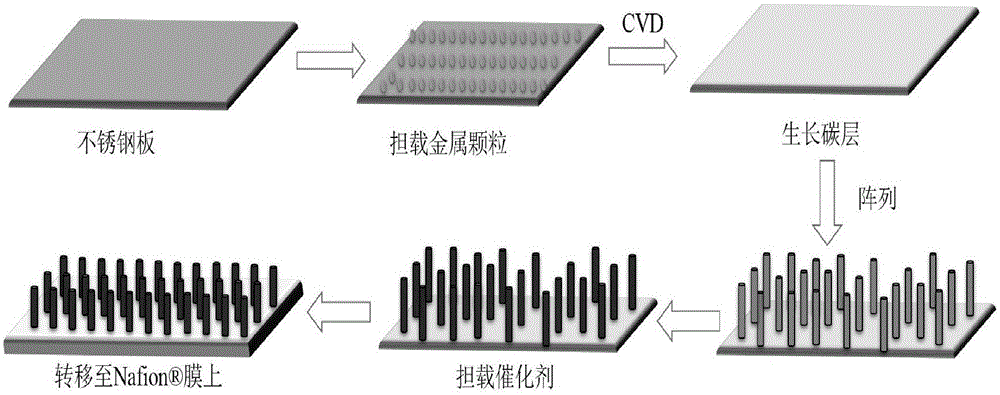
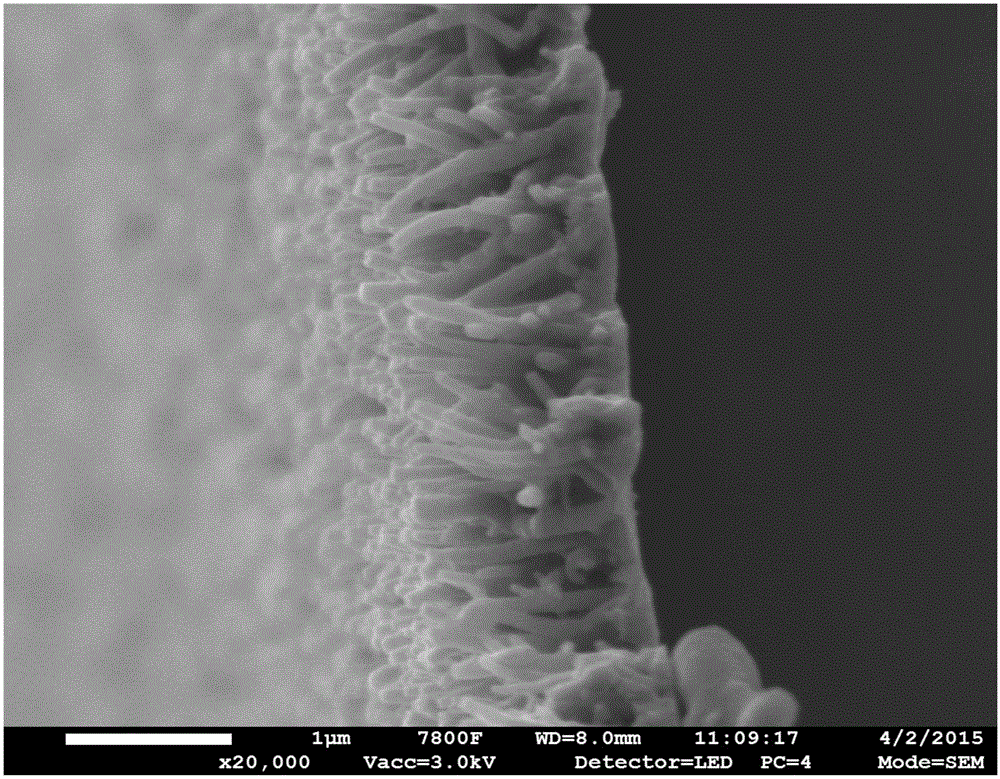
[0024] Step 1: FeCo alloy catalyst loaded on stainless steel surface by magnetron sputtering (co-sputtering FeCo catalyst, deposition conditions: under Ar atmosphere at 20°C, sputtering power 200W, vacuum degree 1.0Pa, sputtering time 6min, Fe : Co (atomic ratio) = 1:1, catalyst thickness: ~ 20nm), and then prepare a carbon layer on the stainless steel surface by CVD (CH 4is the carbon source, the volume ratio of CH 4 :H 2 =1:4, flow rate 50mL min -1 , temperature: 700°C, radio frequency: 200W, reaction time: 25min). Then in situ polymerize the ordered PPy array on the surface of the carbon layer by potentiostatic polymerization (deposition potential 0.7V, temperature: 25°C, reaction time: 20min, reaction solution: 0.2M phosphate buffer + 0.1M p-toluenesulfonate Sodium acid + 0.1M pyrrole monomer), the PPy array has the characteristics of growing perpendicular to the surface of the carbon layer and has a length of 1.2 μm and a diameter of 80 nm.
[0025] Step 2: Deposit Ag...
Embodiment 2
[0028] Take the PPy nanorod array prepared in step 1 of Example 1, and first pass magnetron sputtering Pd catalyst (under Ar atmosphere at 20°C, sputtering power 200W, vacuum degree 1.0Pa, sputtering time Pd: 8min) , and then put the sample in H 2 Saturated 100mL with K 2 PtCl 4 (20mg) ethanol solution, after reduction reaction for 1.5h, the PPy array with the catalyst was transferred onto the Nafion membrane, and used as a single-cell cathode, wherein the cathode catalyst loading (Pt: 0.101mg / cm 2 ,Pd:50μg / cm 2 );
[0029] The anode is commercialized GDE (0.4mg Pt / cm 2 ), used in proton exchange membrane fuel cells. Battery temperature: 65°C, PH 2 =PO 2 =0.05MPa, gas flow: H 2 =50 sccm,O 2 =100sccm, Humidity: H 2 / O 2 =100% / 100%, Nafion membrane.
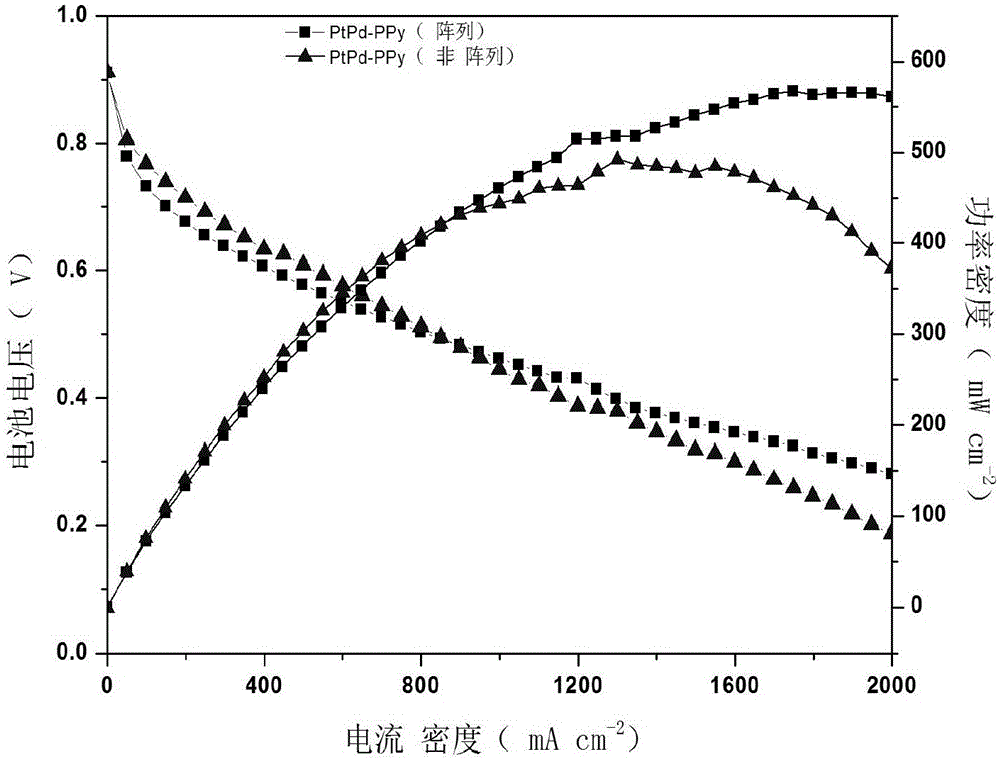
[0030] image 3 is the i_V curve of the single-cell test when the PtPd-PPy ordered electrode is used as the cathode. The single-cell performance of the electrode prepared here is better than that of the previously pr...
Embodiment 3
[0034] Take the PPy nanorod array prepared in step 1 in Example 1.
[0035] Magnetron sputtering Cu metal particles on the PPy nanowire array (Cu: 5min, other sputtering conditions are the same as in Example 1, Cu: 25.245 μg / cm 2 ), by K with 0.1M 2 PtCl 4 The solution was subjected to a displacement reaction to obtain a PtCu-PPy array (Pt: 60.5 μg / cm 2 ).
[0036] The prepared electrode is carried out half-cell test, and the test condition is (N 2 saturated 0.5M H 2 SO 4 , scanning speed 50mV / s, scanning range: -0.241V-0.959V vs SCE)
[0037] Figure 4 It is the CV diagram of PtCu-PPy.
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle diameter | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com