Cefoperazone sodium compound and sulbactam sodium compound prepared with strong-field coupling crystallization technology as well as prepared composition
A technology of cefoperazone sodium and sulbactam sodium, which is applied in the field of medicine and can solve problems affecting the quality of preparations, poor stability, and poor purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0042] Embodiment 1: the preparation of cefoperazone sodium compound
[0043] (1) Dissolve 500g cefoperazone acid in a mixed solvent of 500ml water and 500ml ethanol, and control the temperature at 30°C to dissolve the raw materials;
[0044] (2) Dissolve 65.12g of sodium bicarbonate in 500ml of water, control the reaction temperature at 30°C, slowly add the aqueous solution of sodium bicarbonate into the raw material solution, and stir the reaction until the pH value is 6.0;
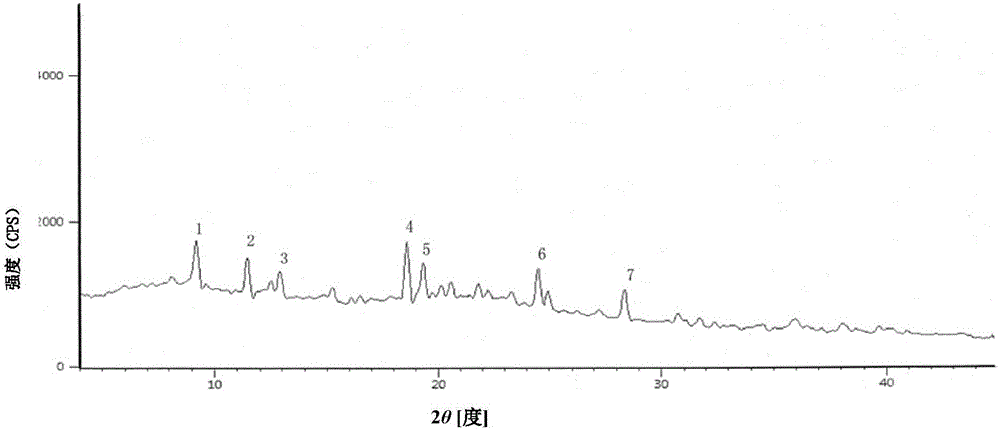
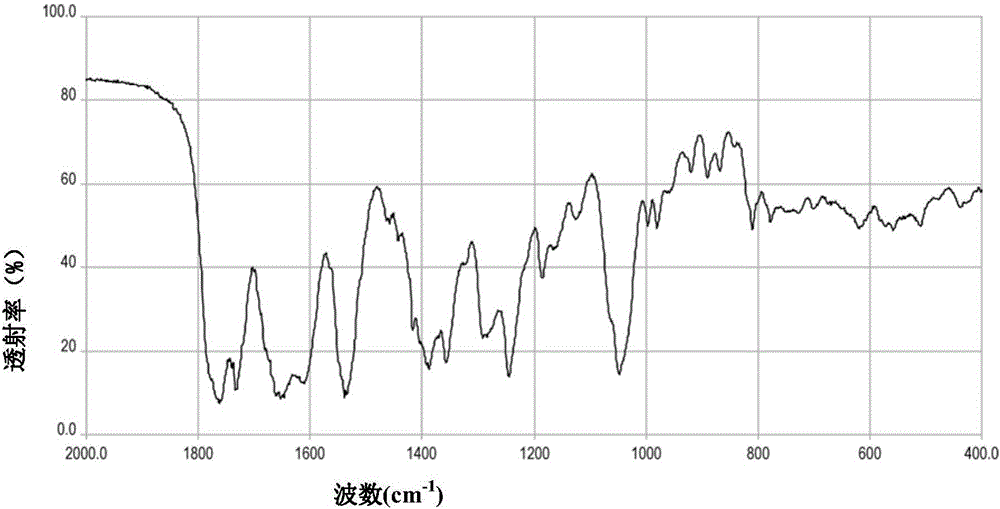
[0045] (3) filter, filtrate is poured in the crystallizer, reduce temperature to 15 ℃, slowly add 2L acetone, lay ultrasonic field in crystallizer interior, carry out the ultrasonic field strengthening of cefoperazone sodium crystallization process, the frequency of ultrasonic field is 100kHz, power is 15W / L solution, filtered, washed the filter cake with acetone, and dried under reduced pressure to obtain 453 g of cefoperazone sodium.
[0046] The X-ray powder diffraction pattern of the product has ch...
Embodiment 2
[0047] Embodiment 2: the preparation of cefoperazone sodium compound
[0048] (1) Dissolve 500g of cefoperazone acid in a mixed solvent of 500ml of water and 750ml of chloroform, and control the temperature at 40°C to dissolve the raw materials;
[0049] (2) Dissolve 104g of sodium lactate in 600ml of water, control the reaction temperature at 40°C, slowly add the aqueous solution of sodium lactate into the raw material solution, and stir the reaction until the pH value is 6.7;
[0050] (3) filter, filtrate is poured in crystallizer, reduce temperature to 20 ℃, slowly add 2L ethyl acetate, lay ultrasonic field in crystallizer interior, carry out the ultrasonic field strengthening of cefoperazone sodium crystallization process, the frequency of ultrasonic field is 20kHz, The power was 5W / L solution, filtered, the filter cake was washed with ethyl acetate, and dried under reduced pressure to obtain 442 g of cefoperazone sodium.
[0051] The X-ray powder diffraction pattern of t...
Embodiment 3
[0052] Embodiment 3: the preparation of sulbactam sodium compound
[0053] (1) Dissolve 650g of 6,6-dibromopenicillane sulfone in a mixed solvent of 500ml of water and 750ml of methanol, and dissolve the raw materials at a temperature of 25°C;
[0054] (2) Dissolve 285g of sodium isooctanoate in water, control the reaction temperature at 25°C, slowly add the aqueous solution of sodium isooctanoate into the raw material liquid, and stir the reaction until the pH value is 4.5;
[0055] (3) Control the temperature at 20°C for carbon adsorption, filter, pour the filtrate into a crystallizer, lower the temperature to 5°C, slowly add acetone, lay out an ultrasonic field inside the crystallizer, and carry out ultrasonication of the crystallization process of sulbactam sodium Field strengthening, the frequency of the ultrasonic field is 20kHz, the power is 5W / L, the solution is filtered, the filter cake is washed with acetone, and dried under reduced pressure to obtain 542g of sulbact...
PUM
| Property | Measurement | Unit |
|---|---|---|
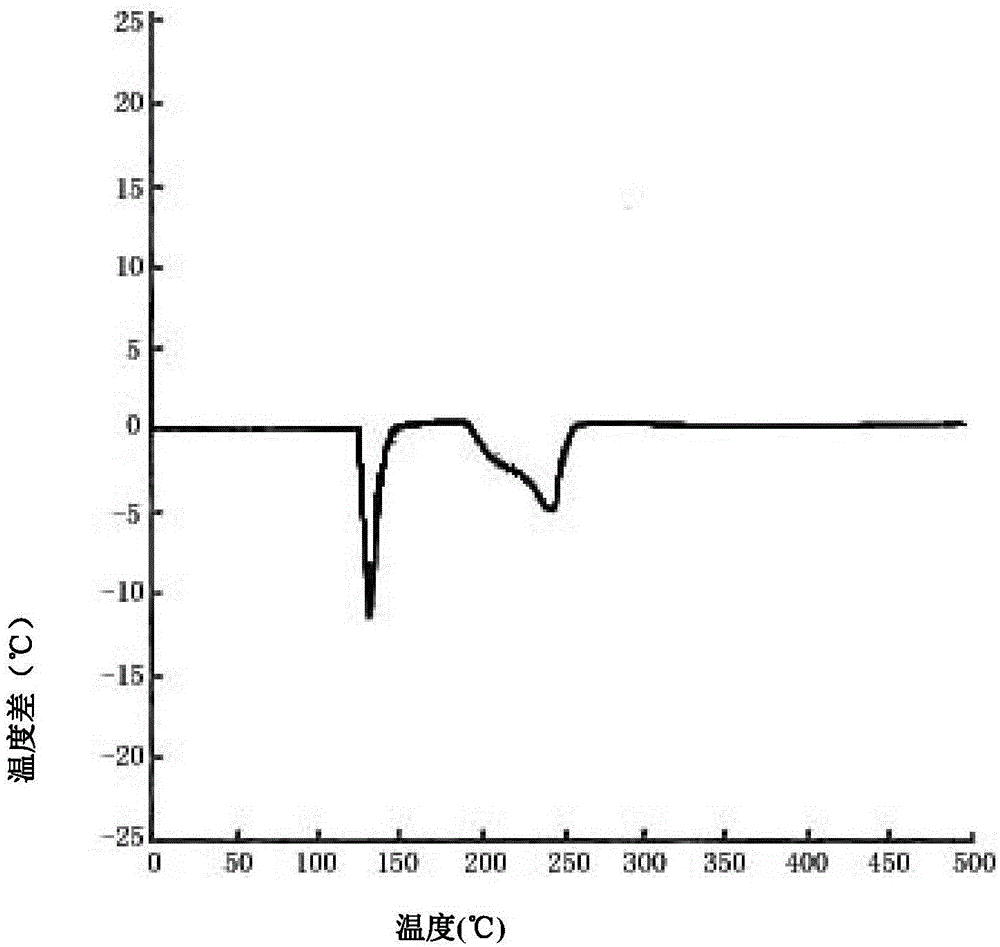
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
| decomposition temperature | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com