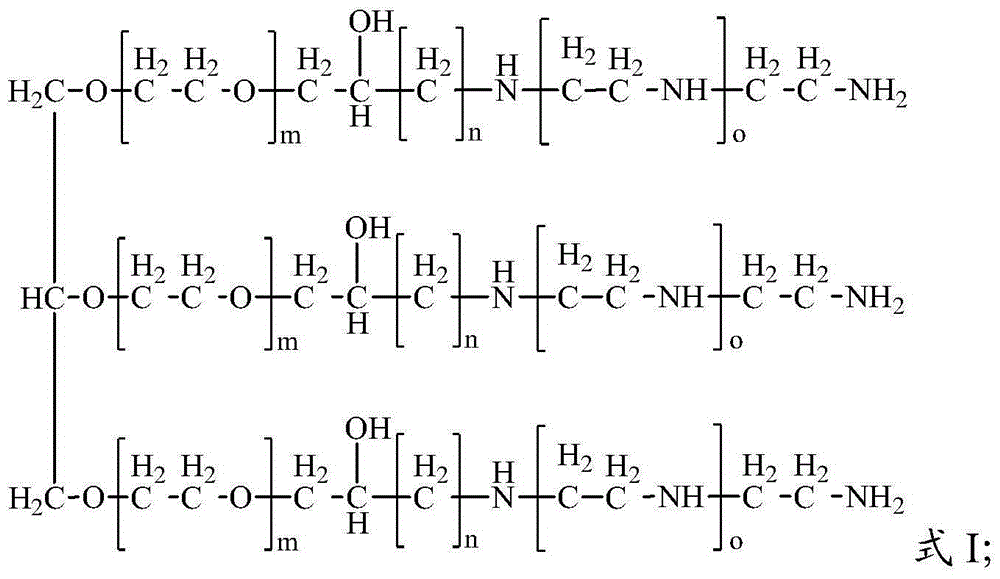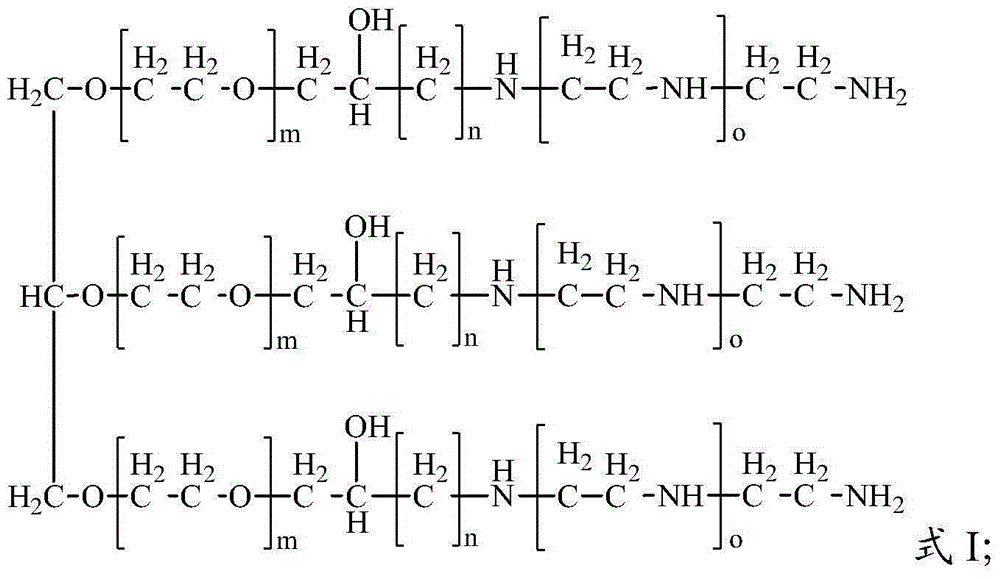Multi-branched polyether amine, preparation method and applications thereof
A polyetheramine chemical and reaction technology, applied in the field of multi-branched polyetheramine and its preparation, can solve the problems of cumbersome polyetheramine reaction steps, low yield of synthetic products, harsh process conditions, etc., and achieve good clay hydration. Dispersion performance, good inhibition performance, simple process effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0029] The present invention provides a kind of preparation method of hyperbranched polyether amine described in above-mentioned technical scheme, comprising:
[0030] reacting glycerol, glycol compounds, water and an acidic catalyst to obtain an intermediate product, the glycol compounds including ethylene glycol or polyethylene glycol;
[0031] React the intermediate product with chlorinated epoxides, basic compounds, and organic amines to obtain hyperbranched polyetheramines; the chlorinated epoxides include epichlorohydrin or 1,2-epoxychlorohydrin Butane, the organic amines include ethylenediamine, diethylenetriamine, triethylenetetramine, tetraethylenepentamine or pentaethylenehexamine.
[0032] In an embodiment of the present invention, the reaction temperature of the glycerin, glycol compounds, water and acidic catalyst is 90°C to 140°C; in other embodiments, the glycerol, glycol compounds , The reaction temperature of water and acidic catalyst is 100℃~120℃. In an emb...
Embodiment 1
[0066] Add 10g of glycerol, 8g of ethylene glycol, 30g of water, and 0.8g of hydrochloric acid into a four-necked flask with a stirring and condensing reflux device, and react at a temperature of 90°C for 2.0h to obtain an intermediate product;
[0067] Add 5g of epichlorohydrin, 0.8g of sodium hydroxide, and 10g of ethylenediamine to the above intermediate product, react at a temperature of 40°C for 0.5h, dry the obtained reaction product to remove water, and obtain a hyperbranched polyether Amine, yield 92.18%.
[0068] The hyperbranched polyetheramine prepared in Example 1 of the present invention has a structure shown in Formula 1:
[0069]
[0070] In Formula 1, m is 1, n is 1, and o is 0.
[0071] According to the method described in the above technical scheme, the primary recovery rate of shale, the relative recovery rate of shale, the relative inhibition rate of clay and the extreme Pressure lubrication coefficient; the test results are shown in Table 1, and Table...
Embodiment 2
[0073] Add 10g of glycerol, 9g of polyethylene glycol with a number average molecular weight of 400, 35g of water, and 0.9g of sulfuric acid into a four-necked flask with a stirring and condensing reflux device, and react at a temperature of 100°C for 3.0h , to get the intermediate product;
[0074] Add 6g of 1,2-epoxychlorobutane, 0.9g of potassium hydroxide, and 11g of diethylenetriamine to the above intermediate product, react at a temperature of 45°C for 1.0h, and dry the obtained reaction product to remove water. A hyperbranched polyetheramine was obtained with a yield of 92.43%.
[0075] The hyperbranched polyetheramine prepared in Example 2 of the present invention has a structure shown in Formula 2:
[0076]
[0077] In Formula 2, m is 9-10, n is 2, and o is 1.
[0078] According to the method described in the above technical scheme, the primary recovery rate of shale, the relative recovery rate of shale, the relative inhibition rate of clay and the extreme The p...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com