A mutant α-amylase and its preparation method and application
A technology of amylase and PET30-PFAMY, which is applied in the field of bioengineering, can solve problems such as poor thermal stability and pH inadaptability, and achieve the effects of simplifying post-processing, improving enzyme activity, and reducing costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
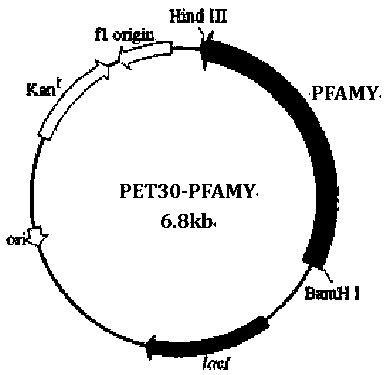
[0034] 1 Gene design and synthesis
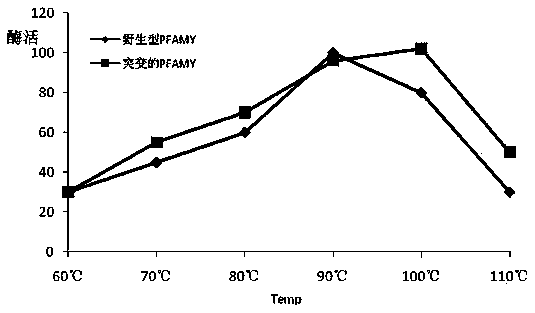
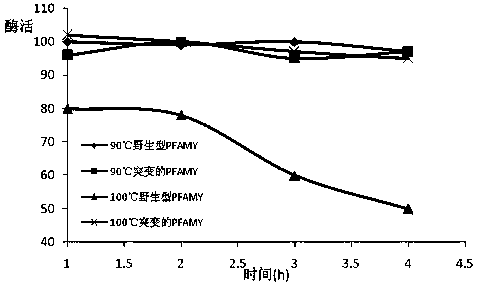
[0035] (1) Gene design: According to the protein sequence of the archaea Pyrococcus furiosus DSM 3638 in Genebank (SEQ ID NO: 1, Genebank: AAL80601.1), mutation sites were introduced into its W65, C204, and N366 sites, and the obtained sequence was as SEQ ID Amino acid sequence shown in NO: 2; use the online design tool Jcat (http: / / www.jcat.de / ) to reverse design the gene sequence. For the preferred codons required for host Escherichia coli expression and the G+C base content required for high-efficiency gene expression, the gene sequence designed above was optimized as SEQ ID NO: 3, which is similar to the wild-type gene sequence SEQ ID NO: 4 Ratio: The number of rare codons in Escherichia coli has been reduced from 13 to 0 in the wild type (SEQ ID NO:4), and the NdeI and SacI restriction sites have been removed during the design process, which is beneficial for later gene manipulation. The above-mentioned serial design is favorable for ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com