A kind of high performance liquid chromatography detection method of sirolimus
A high-performance liquid chromatography and detection method technology, applied in the field of drug analysis, can solve the problems of being unfavorable for the detection of weakly polar and strongly retained impurities, unfavorable for the control of ring-opening degradation products, and not adjusting pH, etc., and achieves good separation and accuracy. High and improved separation effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0048] HPLC detection
[0049] Preparation of sirolimus sample solution: take sirolimus bulk drug, dissolve it with anhydrous acetonitrile, and prepare a 1 mg / mL solution; take 20 μL sirolimus sample solution, inject sample, Qualitative and quantitative analysis of main components, related substances and impurities;
[0050] Wherein, mobile phase A is 20mM ammonium formate buffer solution (i.e. ammonium formate buffer system, pH is about 3.8), mobile phase B is acetonitrile-methyl tert-butyl ether (volume ratio 99:1, the percentage below also represents volume percentage), wherein methyl tert-butyl ether is used as a modifier; the linear gradient elution conditions are: 0~18min: 43%A, 57%B; 18~25min: 29%A, 71%B; 25~33min: 100% B; 33~43min: 100%B (maintain for 10 minutes); after 43min: 43%A, 57%B;
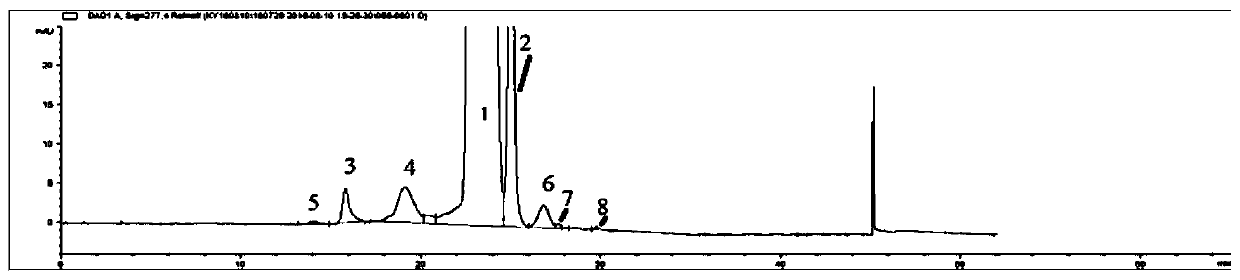
[0051] See Figure 1~4 . exist Figure 1~4 Among them, chromatographic peak 1 is sirolimus, 2 is sirolimus tautomer C, and the rest of the chromatographic peaks are the main impu...
Embodiment 2
[0059] HPLC detection
[0060] Preparation of sirolimus sample solution: take sirolimus capsules from enterprise B, dissolve them in anhydrous acetonitrile, and prepare a 0.5 mg / mL solution; take 20 μL sirolimus sample solution, inject the sample, and Qualitative and quantitative analysis of main components, related substances and impurities in Moss capsules;
[0061] Wherein, mobile phase A is 20mM ammonium formate buffer solution (i.e. ammonium formate buffer system, pH is about 3.8), mobile phase B is acetonitrile-methyl tert-butyl ether (volume ratio 99:1, the percentage below also represents volume percentage), wherein methyl tert-butyl ether is used as a modifier; the linear gradient elution conditions are: 0~18min: 43%A, 57%B; 18~25min: 29%A, 71%B; 25~33min: 100% B; 33~43min: 100%B (maintain for 10 minutes); after 43min: 43%A, 57%B;
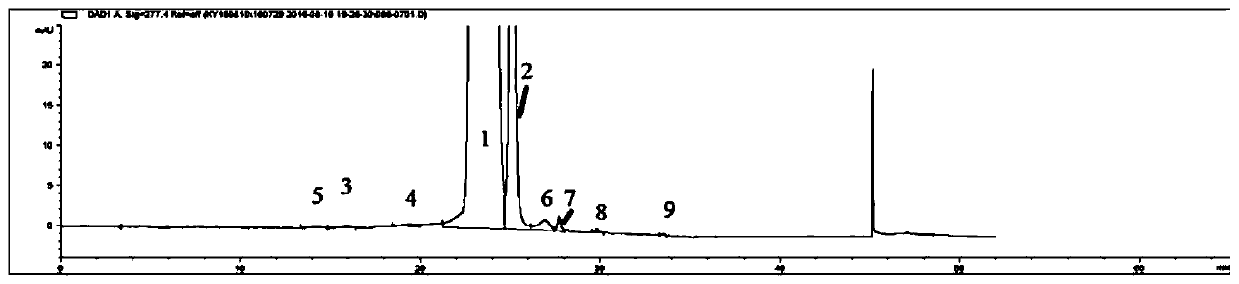
[0062] See the test results Figure 5 , wherein: chromatographic peak 1 is sirolimus, 2 is sirolimus tautomer C, and the remaining chr...
Embodiment 3
[0066] HPLC detection
[0067] Preparation of sirolimus sample solution: take the sirolimus raw material drug from enterprise A, dissolve it in anhydrous acetonitrile, and prepare a 1 mg / mL solution; take 20 μL sirolimus sample solution, inject it, and Qualitative and quantitative analysis of main components, related substances and impurities in Moss capsules;
[0068] Among them, mobile phase A is 20mM ammonium formate buffer solution (i.e. ammonium formate buffer system, pH is about 3.8), mobile phase B is acetonitrile-tetrahydrofuran (volume ratio 97:3), wherein tetrahydrofuran is used as modifier; linear gradient elution The conditions are: 0-20min: 44%A, 56%B; 20-30min: 57%A, 43%B; 30-40min: 100%B; 40-50min: 100%B (maintain for 10 minutes); after 50min : 44% A, 56% B;
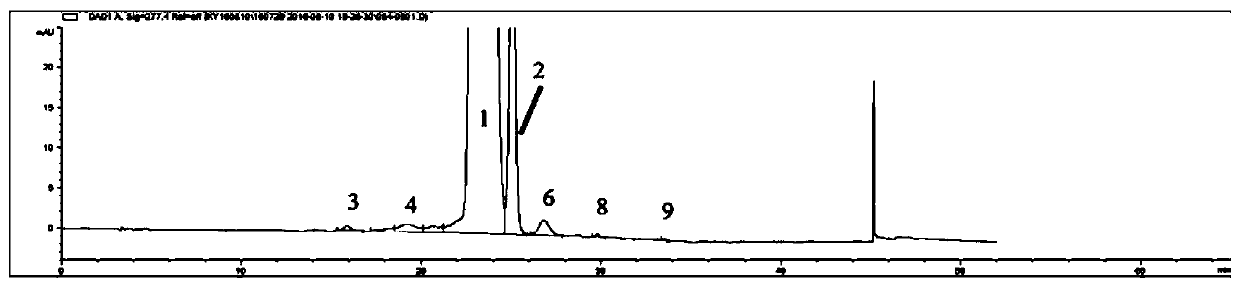
[0069] See the test results Figure 9 , wherein: chromatographic peak 1 is sirolimus, 2 is sirolimus tautomer C, and the rest of the chromatographic peaks are main impurity peaks: 3 is prolyl sirolimus,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com