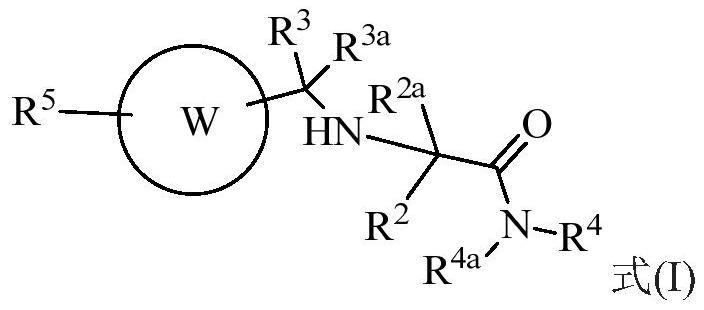
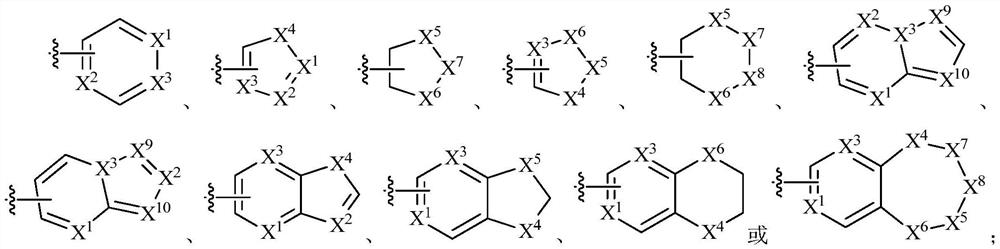
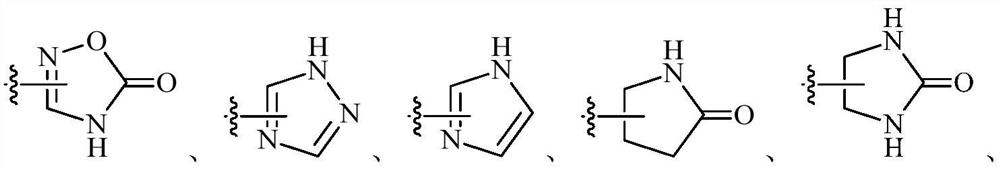
Cathepsin k inhibitors and uses thereof
A compound, an independent technology, applied in anti-inflammatory agents, drug combinations, non-central analgesics, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0217] Example 1N-(1-((1-cyanocyclopropyl)carbamoyl)cyclohexyl)-5-(4-(methylsulfonyl)phenyl)-benzofuran-2-carboxamide
[0218]
[0219] Step 1: Ethyl 5-bromobenzofuran-2-carboxylate
[0220] Add 5-bromo-2-hydroxybenzaldehyde (15g, 74.63mmol), ethyl bromoacetate (18.7g, 111.9mmol), potassium carbonate (31g, 223.9mmol) and anhydrous DMF (80mL) into a 250mL two-necked flask , the reaction mixture was heated to 130° C. for 5 hours to complete the reaction. After filtration, the filtrate was concentrated under reduced pressure, and the residue was dissolved in ethyl acetate (100 mL), washed with saturated brine (50 mL×3), and dried over anhydrous sodium sulfate. After filtration, the solvent was evaporated under reduced pressure, and the crude product was subjected to silica gel column chromatography (ethyl acetate:petroleum ether=1:20, V / V) to obtain 16.6 g of light yellow solid with a yield of 83%.
[0221] MS(ESI,pos.ion)m / z:269.9(M+2);
[0222] Step 2: 5-Bromobenzofuran-2...
Embodiment 2
[0235] Example 2N-(1-((1-cyanocyclopropyl)carbamoyl)cyclohexyl)-6-fluoro-5-(4-(methylsulfonyl)phenyl)-benzofuran-2-methyl Amide
[0236]
[0237] Step 1: Ethyl 5-bromo-4-fluorobenzofuran-2-carboxylate
[0238] Add 5-bromo-4-fluoro-2-hydroxybenzaldehyde (16.3g, 74.63mmol), ethyl bromoacetate (18.7g, 111.9mmol), potassium carbonate (31g, 223.9mmol) and no Water DMF (80mL), prepared according to the synthetic method of Example 1 Step 1, the crude product was subjected to silica gel column chromatography (ethyl acetate:petroleum ether=1:20, V / V) to obtain 16.1g of a light yellow solid, the yield 75%.
[0239] MS(ESI,pos.ion)m / z:287.9(M+2);
[0240] Step 2: 5-Bromo-4-fluorobenzofuran-2-carboxylic acid
[0241] Add ethyl 5-bromo-4-fluorobenzofuran-2-carboxylate (4.26g, 14.86mmol), methanol (50mL) and NaOH solution (22.3mL, 44.6mmol) into a 100mL two-necked flask, according to Example 1. Prepared by the synthetic method of step 2. After vacuum drying, 3.46 g of white solid wa...
Embodiment 3
[0250] Example 3 N-(1-((1-cyanocyclopropyl)carbamoyl)cyclohexyl)-5-(2-oxopyrrolidin-1-yl)benzofuran-2-carboxamide
[0251]
[0252] Step 1: Ethyl 5-bromobenzofuran-2-carboxylate
[0253] Add 5-bromo-2-hydroxybenzaldehyde (15g, 74.63mmol), ethyl bromoacetate (18.7g, 111.9mmol), potassium carbonate (31g, 223.9mmol) and anhydrous DMF (80mL) into a 250mL two-necked flask , was prepared according to the synthesis method in Step 1 of Example 1, and the crude product was subjected to silica gel column chromatography (ethyl acetate:petroleum ether=1:20, V / V) to obtain 16.6 g of a light yellow solid with a yield of 83%.
[0254] MS(ESI,pos.ion)m / z:269.9(M+2);
[0255] Step 2: 5-Bromobenzofuran-2-carboxylic acid
[0256] Add ethyl 5-bromobenzofuran-2-carboxylate (4g, 14.86mmol) and methanol (50mL) into a 100mL two-necked flask, then add NaOH solution (22.3mL, 44.6mmol), according to step 2 of Example 1 Prepared by the synthetic method, obtained white solid 3.30g after vacuum dryin...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com