Pyrazolyloxadiazole thioether compounds, and preparation method and application thereof
A technology of pyrazole-bioxadiazole sulfide and azole-bioxadiazole sulfide, which is applied in the field of medicinal chemistry, can solve the problem of aggravating the accumulation of poisonous sources and spreading in a large area, and cannot effectively prevent and control plant virus diseases, harm, etc. problems, to achieve good therapeutic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
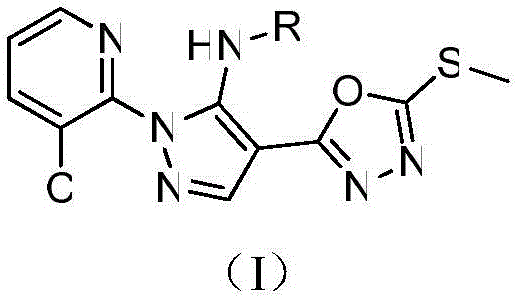
[0014] Example 1 of the present invention: target compound 1-(3-chloropyridin-2-yl)-N-methyl-4-(5-(methylthio)-1,3,4-oxadiazole-2 Preparation of -yl)-1H-pyrazol-5-amine
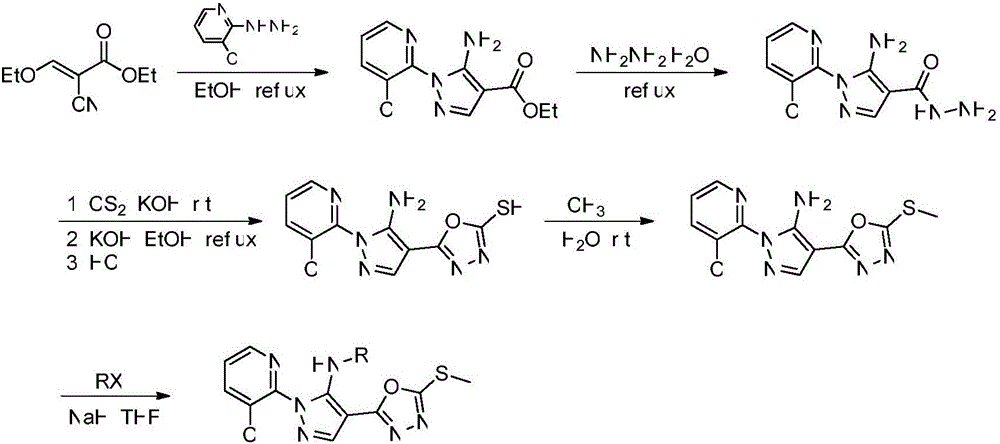
[0015] Add the intermediate 5-amino-1-(3-chloro-2-pyridyl)-4-(5-methylthio-1,3,4-oxadiazol-2-yl)- Pyrazole (1.1mmol), sodium hydride (2.27mmol) and 10mL of anhydrous tetrahydrofuran, methyl iodide (1.3mmol) were slowly added to the reactor, reacted at room temperature for 5h, TCL detected the end of the reaction, and the solvent was removed by distillation under reduced pressure. The target compound was separated and purified by column chromatography [eluent (v / v): petroleum ether / ethyl acetate=40 / 1] as a brown solid with a yield of 40.5% and a melting point of 97-98°C.
[0016] The synthesis of the target compound substituted with alkanes and alkenes refers to Example 1.
Embodiment 2
[0017] Example 2 of the present invention: target compound 1-(3-chloropyridin-2-yl)-N-benzyl-4-(5-(methylthio)-1,3,4-oxadiazole-2 Preparation of -yl)-1H-pyrazol-5-amine
[0018] Add the intermediate 5-amino-1-(3-chloro-2-pyridyl)-4-(5-methylthio-1,3,4-oxadiazol-2-yl)- Pyrazole (1.1mmol), sodium hydride (2.27mmol) and 10mL of anhydrous tetrahydrofuran, methyl iodide (1.3mmol) were slowly added to the reactor, reacted at room temperature for 5h, TCL detected the end of the reaction, and the solvent was removed by distillation under reduced pressure. The target compound was separated and purified by column chromatography [eluent (v / v): petroleum ether / ethyl acetate=40 / 1] as a white solid with a yield of 51.6% and a melting point of 154-155°C.
[0019] Refer to Example 2 for the synthesis of target compounds substituted by benzyl, substituted benzyl and heterocycles.
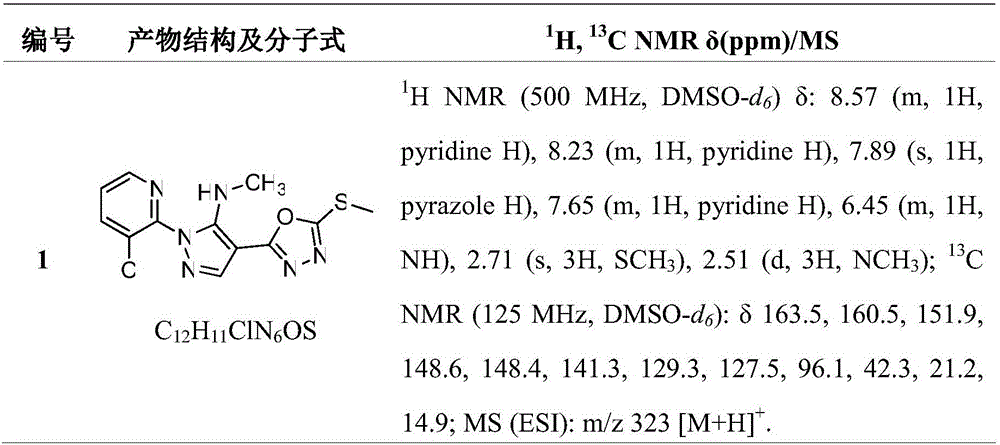
[0020] The structures, H-NMR, C-NMR and mass spectrometry data of some of the synthesized pyrazole-oxadiazole s...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com