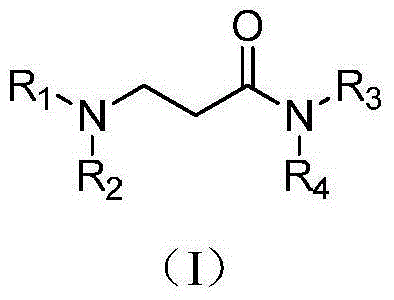
3-(N, N-disubstituted amino) propanamide derivative and preparation method and use thereof in medicine
A double substitution and propionamide technology, applied in the field of chemical synthesis of drugs, can solve the problems of clinical research failure, failure to produce high affinity, and inability to reduce LDL-C levels
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
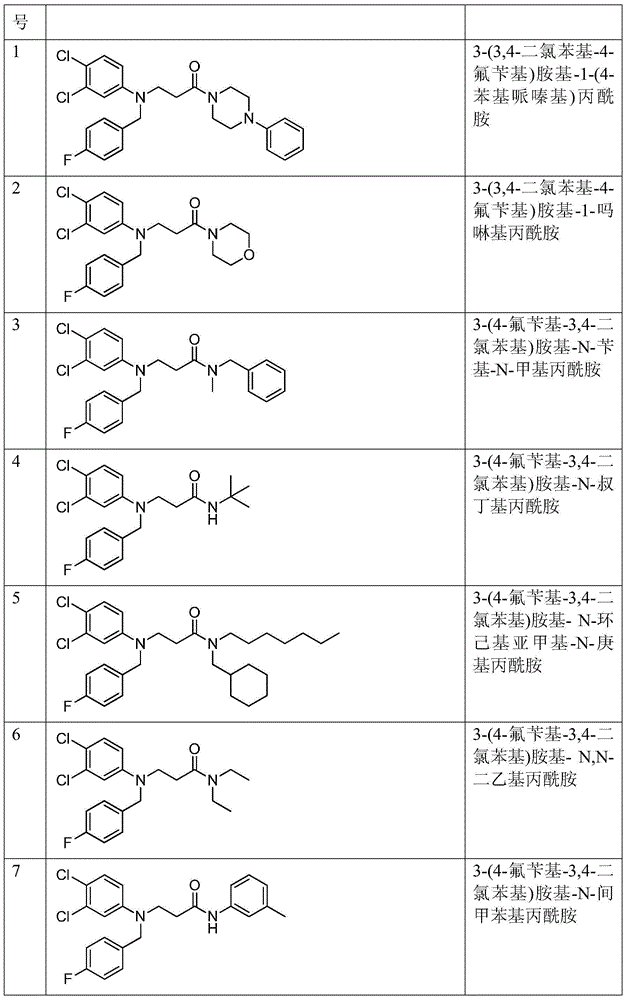
[0057] Example 1: Preparation of 3-(3,4-dichlorophenyl-4-fluorobenzyl)amino-1-(4-phenylpiperazinyl)propanamide
[0058] (1) Preparation of 3-(3,4-dichlorophenyl)aminoacrylic acid:
[0059] Add 3,4-dichloroaniline (4.6g) into a 50mL three-necked flask, add (17.0mL) acrylic acid and 10% hydrochloric acid respectively, heat to 50°C, and react for 3h. Heating was stopped, and the reaction solution was slowly poured into a large amount of ice-water mixture, a large amount of light yellow solid was precipitated, which was suction filtered and vacuum-dried to constant weight. After column chromatography (petroleum ether: ethyl acetate: glacial acetic acid = 5:1:0.01), a yellow solid 3-(3,4-dichlorophenyl)aminoacrylic acid (3.5g) was obtained with a yield of 72.10% . Melting point: 49.5-51.2°C.
[0060] (2) Preparation of 1-phenylpiperazine:
[0061] In a 50mL three-neck flask, dissolve 2.1g (20mmol) of diethanolamine in 15mL of dichloromethane, dissolve 7mL (excess) of thionyl ch...
Embodiment 2
[0068] Example 2: Preparation of 3-(3,4-dichlorophenyl-4-fluorobenzyl)amino-1-morpholinopropionamide
[0069] The preparation process of compound 2 is the same as that of compound 1. 1 H-NMR (400MHz, CDCl 3 , ppm) δ: 7.21-7.07 (m, 3H), 7.04-6.93 (m, 2H), 6.73 (d, J = 2.97Hz, 2H), 6.49 (dd, J = 2.97, 8.97Hz, 1H), 4.51 (s,1H),3.77(t,J=7.08Hz,2H),3.69-3.55(m,6H),3.40-3.33(m,2H),2.58(d,J=6.93Hz,2H); ESI- MS(m / z):411.1[M+H] + ,433.1[M+Na] + .
Embodiment 3
[0070] Example 3: Preparation of 3-(4-fluorobenzyl-3,4-dichlorophenyl)amino-N-benzyl-N-methylpropionamide
[0071] The preparation process of compound 3 is the same as that of compound 1. 1 H-NMR (400MHz, CDCl 3 )δ: 7.37-7.27 (m, 3H), 7.24-7.03 (m, 5H), 7.04-6.90 (m, 2H), 6.72 (dd, J=2.94, 21.2Hz, 1H), 6.57-6.36 (m, 1H), 4.60-4.40(m, 4H), 3.89-3.74(m, 2H), 3.03-2.97(m, 3H), 2.72-2.58(m, 2H); ESI-MS(m / z): 445.2[ M+H] + ,467.2[M+Na] + ,483.2[M+K] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| Melting point | aaaaa | aaaaa |
| Melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com