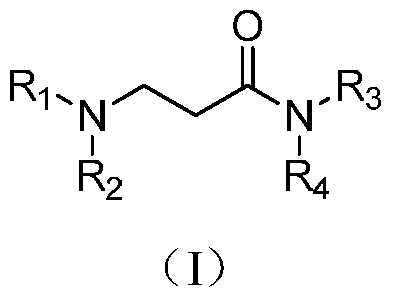
3-(n,n-disubstituted amino)propionamide derivatives, preparation method and application in medicine
A propionamide, amine-based technology, used in the field of chemical synthesis of drugs, can solve the problems of clinical research failure, no high affinity, and inability to reduce LDL-C levels.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
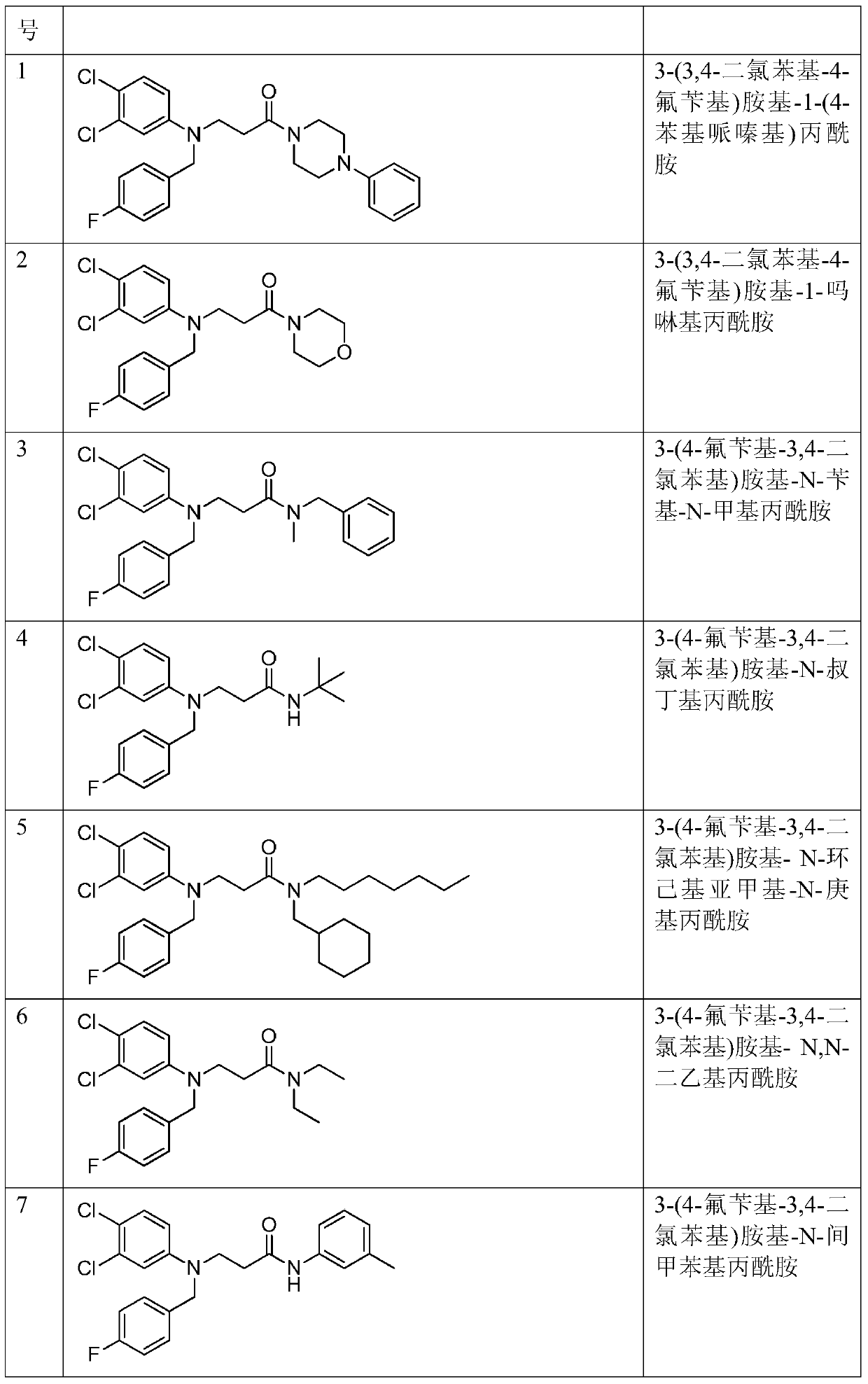
[0055] Example 1: Preparation of 3-(3,4-dichlorophenyl-4-fluorobenzyl)amino-1-(4-phenylpiperazinyl)propanamide
[0056] (1) Preparation of 3-(3,4-dichlorophenyl)aminoacrylic acid:
[0057] Add 3,4-dichloroaniline (4.6g) into a 50mL three-necked flask, add (17.0mL) acrylic acid and 10% hydrochloric acid respectively, heat to 50°C, and react for 3h. Heating was stopped, and the reaction solution was slowly poured into a large amount of ice-water mixture, a large amount of light yellow solid was precipitated, which was suction filtered and vacuum-dried to constant weight. After column chromatography (petroleum ether: ethyl acetate: glacial acetic acid = 5:1:0.01), a yellow solid 3-(3,4-dichlorophenyl)aminoacrylic acid (3.5g) was obtained with a yield of 72.10% . Melting point: 49.5-51.2°C.
[0058] (2) Preparation of 1-phenylpiperazine:
[0059] In a 50mL three-neck flask, dissolve 2.1g (20mmol) of diethanolamine in 15mL of dichloromethane, dissolve 7mL (excess) of thionyl ch...
Embodiment 2
[0066] Example 2: Preparation of 3-(3,4-dichlorophenyl-4-fluorobenzyl)amino-1-morpholinopropionamide
[0067] The preparation process of compound 2 is the same as that of compound 1. 1 H-NMR (400MHz, CDCl 3 , ppm) δ: 7.21-7.07 (m, 3H), 7.04-6.93 (m, 2H), 6.73 (d, J = 2.97Hz, 2H), 6.49 (dd, J = 2.97, 8.97Hz, 1H), 4.51 (s,1H),3.77(t,J=7.08Hz,2H),3.69-3.55(m,6H),3.40-3.33(m,2H),2.58(d,J=6.93Hz,2H); ESI- MS(m / z):411.1[M+H] + ,433.1[M+Na] + .
Embodiment 3
[0068] Example 3: Preparation of 3-(4-fluorobenzyl-3,4-dichlorophenyl)amino-N-benzyl-N-methylpropionamide
[0069] The preparation process of compound 3 is the same as that of compound 1. 1 H-NMR (400MHz, CDCl 3 )δ: 7.37-7.27 (m, 3H), 7.24-7.03 (m, 5H), 7.04-6.90 (m, 2H), 6.72 (dd, J=2.94, 21.2Hz, 1H), 6.57-6.36 (m, 1H), 4.60-4.40(m, 4H), 3.89-3.74(m, 2H), 3.03-2.97(m, 3H), 2.72-2.58(m, 2H); ESI-MS(m / z): 445.2[ M+H] + ,467.2[M+Na] + ,483.2[M+K] + .
PUM
| Property | Measurement | Unit |
|---|---|---|
| melting point | aaaaa | aaaaa |
| melting point | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com