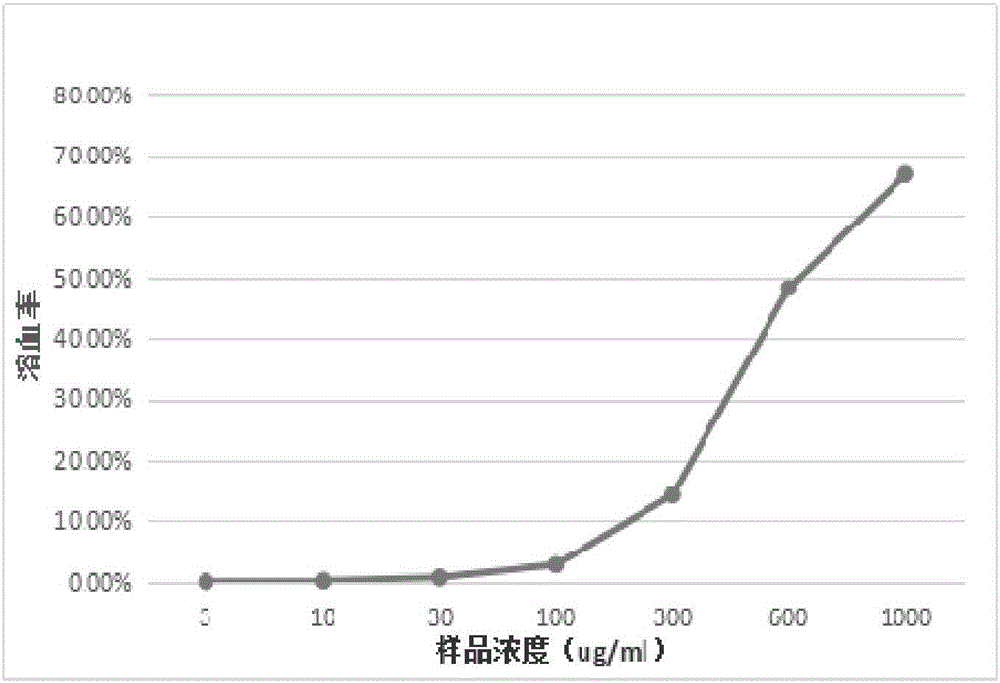
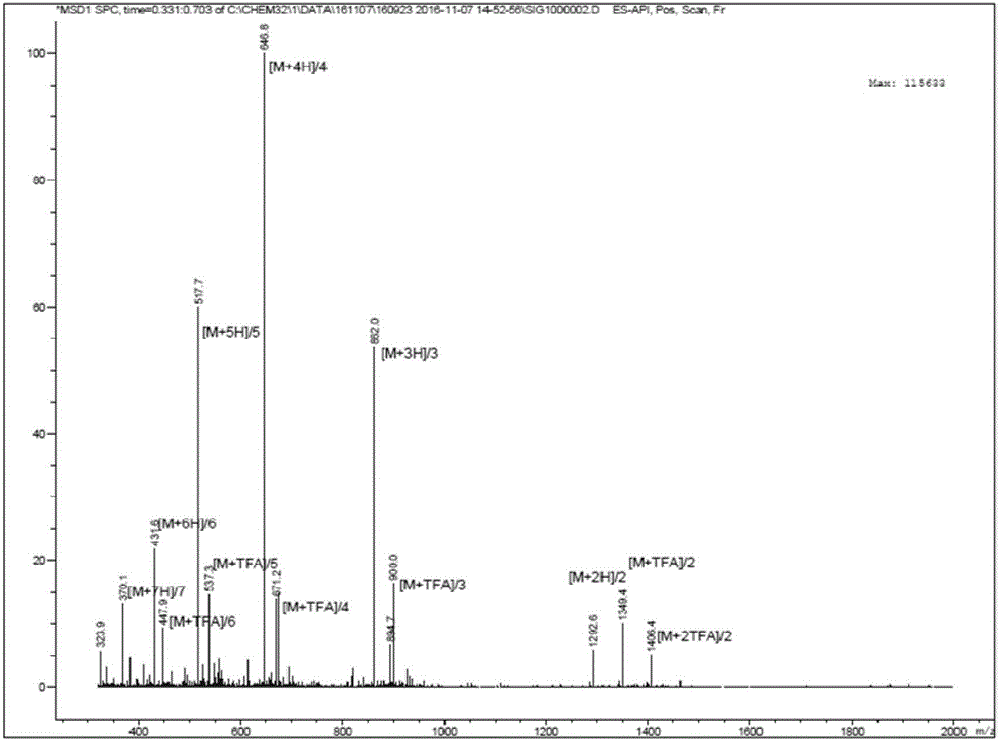
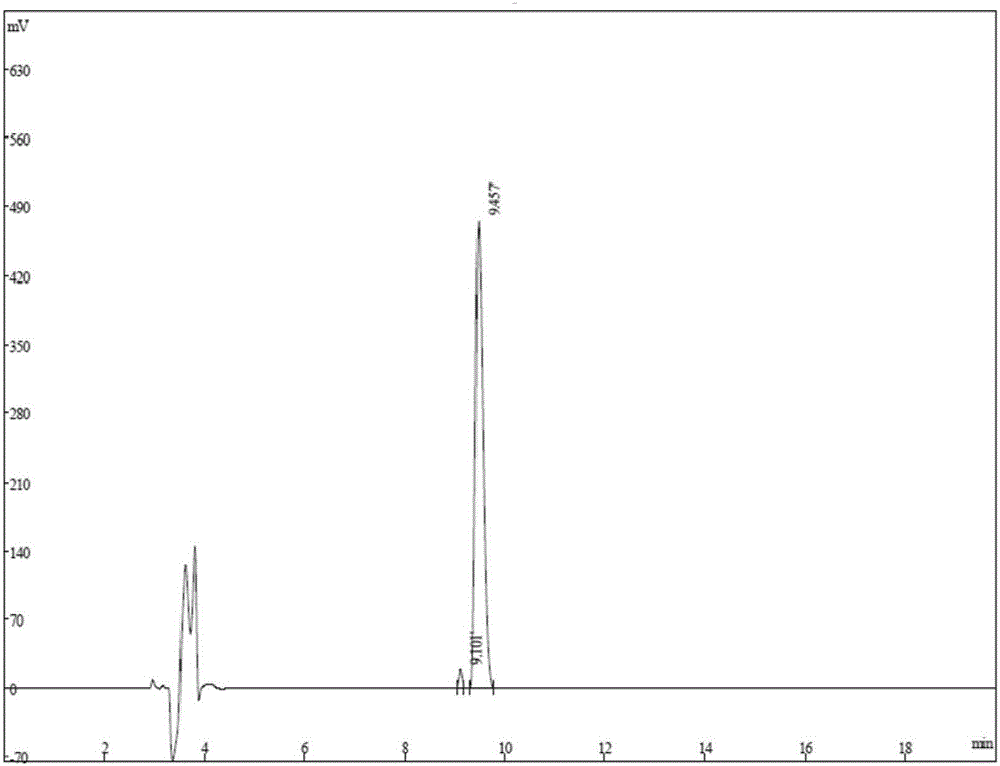
Antimicrobial peptide
A technology of antimicrobial peptides and compositions, applied in the field of antimicrobial peptides, which can solve the problems of toxicity, poor stability, and low antibacterial activity of antimicrobial peptides
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0023] Example 1. Synthesis and purification of antimicrobial peptides
[0024] 1. According to the Fmoc solid-phase peptide synthesis method, the antimicrobial peptide BT315 whose X1 is Arg and X2 is Lys is synthesized. The synthesis steps are as follows:
[0025] 1.1 To ensure that the C-terminus of the polypeptide is -CONH 2 Structure, choose Rink Amide resin as polymer solid phase carrier.
[0026] 1.2 Remove the Fmoc protecting group:
[0027] 1.2.1 Put the required polymer solid phase carrier into the synthesis column, add the organic solvent DMF (dimethylformamide) to soak for 10 minutes and then discharge; pass into 20% piperidine / DMF solution to cover the resin surface, pass through N 2 Discharge after reacting for 10 minutes; repeat to feed 20% piperidine / DMF, 2 Discharge after 10 minutes of reaction.
[0028] 1.2.2N 2 Under protection, pass through DMF solvent 5 times to thoroughly wash the above resin.
[0029] 1.3 Condensation reaction:
[0030] Amino Acid...
Embodiment 2
[0052] Embodiment 2. The minimum inhibitory concentration (MIC) and the minimum bactericidal concentration (MBC) of antimicrobial peptide BT315 are measured
[0053] The antimicrobial peptide BT31 prepared in Example 1 was diluted 15 times with sterile culture solution to make 1 mL concentrations of 512, 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25 μg / mL antimicrobial peptide solution. Inoculate the tested strain into 2mL culture medium, culture at 37°C for 8h, and dilute with sterile culture medium to obtain a colony count of approximately 5×10 5 About CFU / mL bacterial liquid. Take 1mL of bacterial liquid and add them to the prepared antimicrobial peptide solutions above. At this time, the concentrations of antimicrobial peptides in each solution are 256, 128, 64, 32, 16, 8, 4, 2, 1, 0.5, 0.25, 0.125 μg / mL. Antibiotics vancomycin and imipenem solutions were prepared in the same way as positive controls; another drug-free group was set as negative controls. Incubate in an o...
Embodiment 3
[0058] The influence of embodiment 3.pH value on the MIC value of antimicrobial peptide BT315
[0059] Adjust the pH of the culture medium containing different sample concentrations to 5.0, 6.0, 7.0, 8.0, 9.0 respectively, and after standing at room temperature for 1 hour, adjust the pH value to 7.0, and then use sterile medium to adjust the volume to be consistent, according to the example The method described in 2 determines the MIC value. The results are shown in Table 2 below.
[0060] The minimum inhibitory concentration (MIC) of table 2 antimicrobial peptide BT315 at different pH
[0061]
[0062] It can be seen from the above table that the antibacterial peptide BT315 has relatively stable antibacterial activity at different pH values, and the activity is the highest at neutral pH. This indicated that the antimicrobial peptide BT315 had better stability.
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com