Use of compositions comprising bifidobacterium animalis ssp. lactis LMG P-28149
A technology of LMGP-28149, animal bifidobacteria, applied in the direction of bifidobacteria, drug combination, microorganism-based methods, etc., can solve the problem of maintaining the expected normal level
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0150] Example 1: Ls33 and mixture
[0151] This example shows the effect of Ls33 on the development of overweight and obesity in mice.
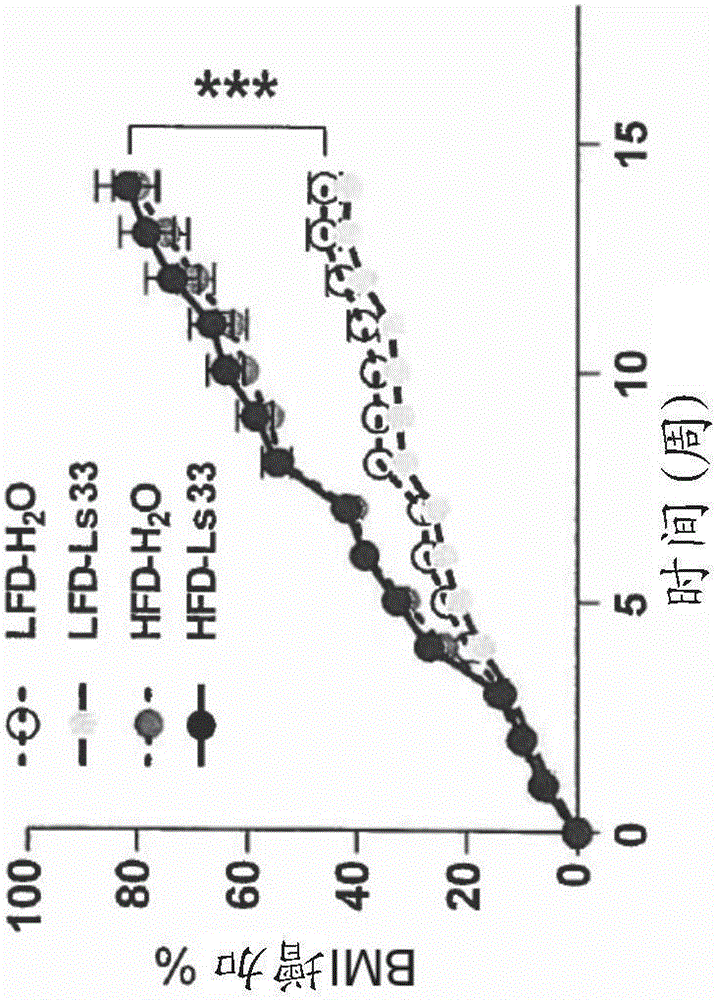
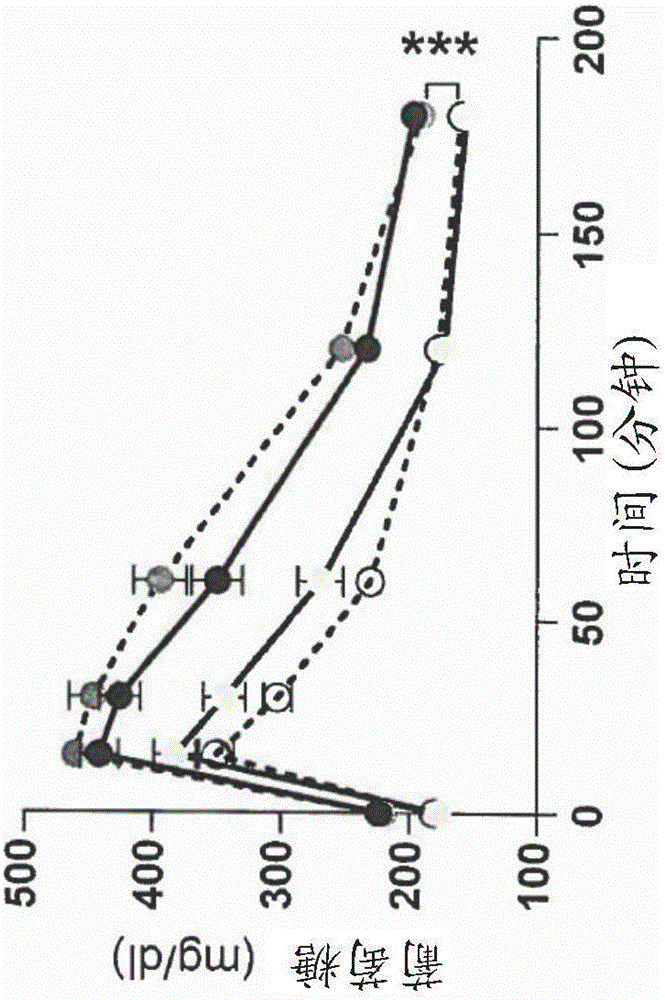
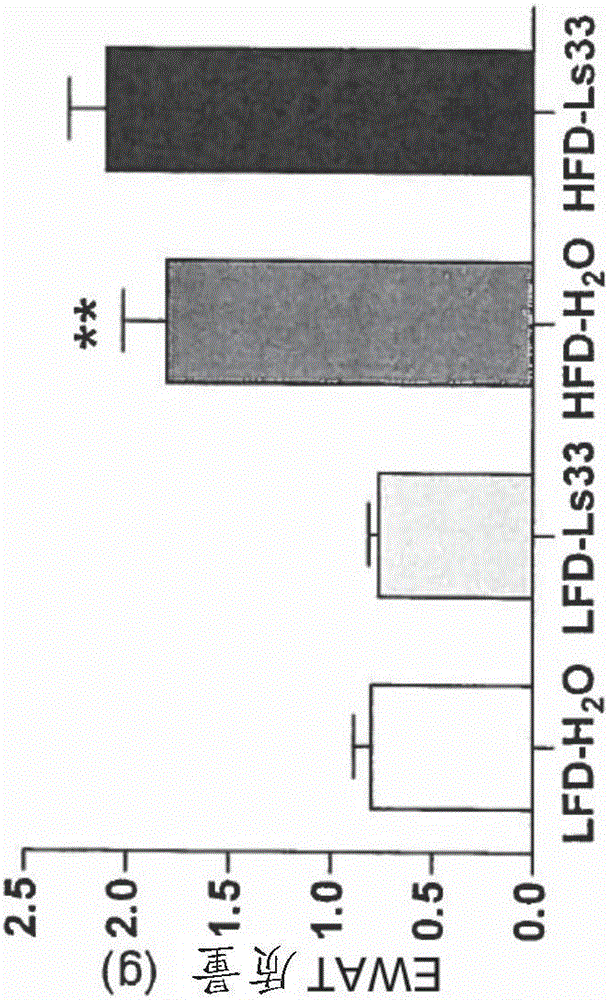
[0152] Figure 1 shows that for 15 weeks of treatment in Ls33 or water and food diet with low fat level (LFD) or high fat level (HFD), the following indications:
[0153] (A) Change in body weight gain over time expressed as a percentage relative to body weight measured on d=0 day;
[0154] (B) Glucose tolerance test (GTT) performed after 12 weeks of diet. Glucose levels (mg / dl) in mice were measured after a 6-hour fasting period, at the time t (minutes) indicated on the graph, after intraperitoneal (IP) injection of glucose (corresponding to time t=0).
[0155] (C) Weight (volume) (g) of epididymal adipose tissue (EWAT) after 15 weeks of diet (weighed during sacrifice);
[0156] (D) Subcutaneous adipose tissue (SCWAT) weight (volume) (g) after 15 weeks of diet (weighed during sacrifice).
[0157] As the results shown in this figure, st...
Embodiment 2
[0173] Example 2: Effects of Mix on inflammation of white adipose tissue
[0174] Figure 3 shows the effect of Mix on:
[0175] (A) Weight (quantity) (g) of EWAT;
[0176] (B) Weight (quantity) (g) of SCWAT;
[0177] (C) The amount of leptin (ng / ml) in the blood as measured by ELISA (enzyme-linked immunoassay) in the serum of mice fasted for 6 hours;
[0178] (D) The amount of adiponectin in the blood (μg / ml) as measured by ELISA in the serum of mice fasted for 6 hours;
[0179] (E) Histology of epididymal white adipose tissue (hematoxylin and eosin stained sections representative of each experimental group are presented). Scale bar is 100 μm. Black arrows indicate the presence of cellular infiltration; and
[0180] (F) Size distribution of adipocytes in EWAT. Results are expressed as % of adipocytes in each size class (0-20 [mu]m, 20-40, etc.). At the same time, Figure 7 shows the effect of Mix on the following aspects:
[0181] (A) Weight (amount) (g) of pancreas, ...
Embodiment 3
[0197] Example 3: Effects of Mix on lipid metabolism and short-chain fatty acid (SCFA) production in the small intestine
[0198] In this example, it was shown that the expression levels of genes (GPR41 and GPR43) responsible for the transport of short-chain fatty acids (Short-Chain Fatty Acid, SCFA) were reduced in the small intestine of mice on the HFD diet, which indicated that in the HFD group, While the latter was less detected, SCFA was able to play a positive role to a lesser extent, while genes (GPR41 and GPR43) were significantly increased in Mix-treated animals under the HFD diet (see Figure 5B )
[0199] In turn, administration of Mix had the effect of limiting increases in genes essential in fatty food diet-induced lipid metabolism (see Figure 5A ).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com