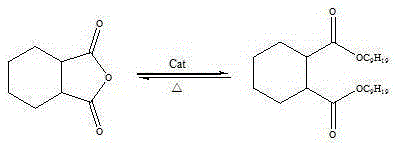
Preparation method for diisononyl cyclohexane-1,2-dicarboxylate
A technology of diisononyl dicarboxylate and cyclohexane, which is applied in the field of fine chemicals, can solve the problems of shortening the reaction time, increasing the burden on enterprises, and high product color, so as to shorten the reaction time, improve production efficiency and yield, good catalytic activity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
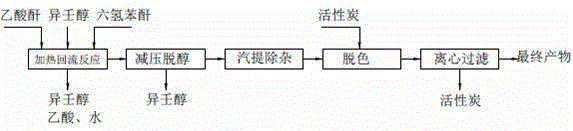
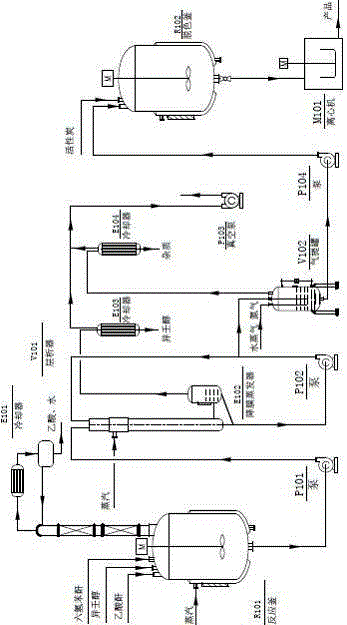
[0034] Add hexahydrophthalic anhydride, isononanol, and catalyst acetic anhydride in proportion to the reactor, wherein: the molar ratio of hexahydrophthalic anhydride to isononanol is 1:2.3, and the amount of acetic anhydride added is 1 / 2 of the quality of hexahydrophthalic anhydride 5%, heated and stirred under normal pressure, raised the temperature to 180°C and refluxed to start timing, continued to heat up the reaction, and kept at 230°C for 3 hours to obtain material 1, which was crude diisononyl cyclohexane-1,2-dicarboxylate ; The material 1 is passed through the falling film evaporator, and the excessive alcohol is flashed off under the negative pressure of -0.06MPa, and the residence time is 0.002 h to obtain the material 2, which enters the stripping tank; in the stripping tank, feed water vapor and Nitrogen gas is bubbled, and the low molecular weight impurities in the material 2 are evaporated to obtain the material 3, which is put into the decolorization kettle. T...
Embodiment 2
[0041] Add hexahydrophthalic anhydride, isononanol, and catalyst acetic anhydride in proportion to the reactor, wherein the molar ratio of hexahydrophthalic anhydride to isononanol is 1:2.1, and the amount of acetic anhydride added is 1 / 2 of the quality of hexahydrophthalic anhydride 2%, heated and stirred under normal pressure, raised the temperature to 180°C and refluxed to start timing, continued to heat up the reaction, and obtained material 1 after maintaining at 220°C for 3 hours, material 1 was crude diisononyl cyclohexane-1,2-dicarboxylate ; The material 1 is passed through the falling film evaporator, and the excess alcohol is flashed off under the negative pressure of -0.08MPa, and the residence time is 0.001 h to obtain the material 2, which enters the stripping tank; in the stripping tank, feed water vapor and Nitrogen gas is bubbled, and the low molecular weight impurities in the material 2 are evaporated to obtain the material 3, which is put into the decolorizati...
Embodiment 3
[0043] Put hexahydrophthalic anhydride, isononanol, and catalyst acetic anhydride into the reaction kettle in proportion, wherein: the molar ratio of hexahydrophthalic anhydride to isononanol is 1:2.5, and the amount of acetic anhydride added is 1 / 2 of the quality of hexahydrophthalic anhydride 6%, heated and stirred under normal pressure, raised the temperature to 180°C and refluxed to start timing, continued to raise the temperature to react, and obtained material 1 after maintaining at 250°C for 5 hours, and material 1 was crude diisononyl cyclohexane-1,2-dicarboxylate ; The material 1 is passed through the falling film evaporator, and the excessive alcohol is flashed off under the negative pressure of -0.096MPa, and the residence time is 0.001 h to obtain the material 2, which enters the stripping tank; in the stripping tank, feed water vapor and Nitrogen gas is bubbled, and the low molecular weight impurities in the material 2 are evaporated to obtain the material 3, which...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com