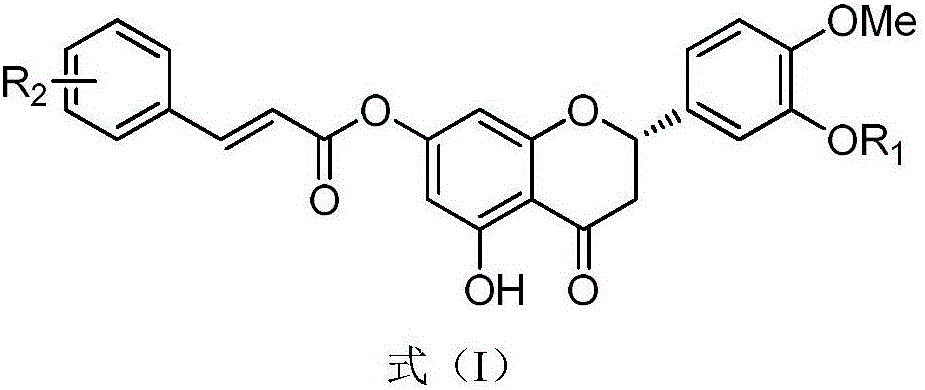
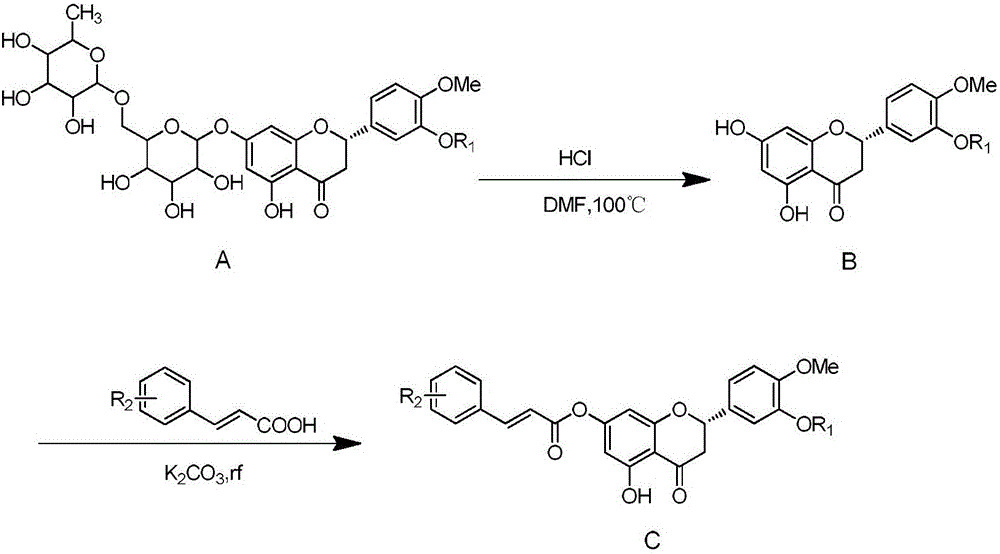
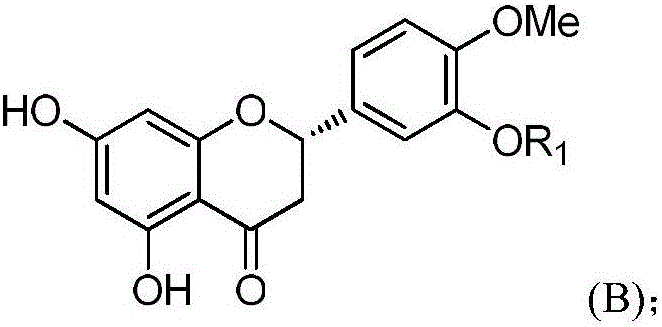
Hesperetin cinnamate compound with anti-tumor activity and synthetic method thereof
A technology of cinnamic esters with anti-tumor activity, applied in the field of medicinal chemistry, can solve problems such as treatment failure, and achieve the effects of low production cost, high operation safety, and full utilization of reaction raw materials
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Preparation of compound (S)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-oxochroman-7-ylcinnamic acid (I-1);
[0037] 100 g of hesperidin and 45.4 g of potassium carbonate were dissolved in dimethylformamide at 10° C. and stirred at 90° C. for 6 hours. The reacted solution was cooled to room temperature. To the solution was added 100 g of iodomethane, followed by stirring at room temperature for 8 hours. After the reaction was completed, 5 L of a mixture of ethyl acetate and dichloromethane (3:2) was added thereto. The resulting solution was stirred for 2 hours, and the precipitated solid was collected by filtration and washed with a small amount of ethanol to obtain the target compound (yellow solid, 40.1 g, yield: 81.5%), whose structural formula is shown in formula (I-1).
[0038]
[0039] 1 H-NMR(300MHz,DMSO)δ:11.35(1H,m),7.60(2H,m),7.48(1H,s),7.40(2H,m),7.33(1H,m),6.99(1H,m ),6.81-6.75(2H,J=7.5,m),6.52-6.48(2H,m),6.31(1H,s),5.51(1H,s),5.35(1H,s),3.83(3H,s ),3....
Embodiment 2
[0041] Compound (S,E)-5-Hydroxy-2-(3-Hydroxy-4-methoxyphenyl)-4-oxochroman-7-yl 3-(p-tolyl)acrylate Preparation of (I-2).
[0042] 100 g of hesperidin and 45.4 g of potassium carbonate were dissolved in dimethylformamide at 10° C. and stirred at 90° C. for 6 hours. The reacted solution was cooled to room temperature. To the solution was added 100 g of iodomethane, followed by stirring at room temperature for 8 hours. After the reaction was completed, 5 L of a mixture of ethyl acetate and dichloromethane (3:2) was added thereto. Add aluminum chloride again, pass into chlorine gas, the obtained solution is stirred for 2 hours, and the precipitated solid is collected by filtration, and washed with a small amount of ethanol to obtain the target compound (light yellow solid, 41.1g, yield: 83.5%), its structural formula is as follows Formula (I-2) shown.
[0043]
[0044] 1 H-NMR(300MHz,DMSO)δ:11.35(1H,m),7.68(2H,m),7.48(1H,s),7.44(2H,m),6.99(1H,m),6.85-6.71(2H ,J=7.5,m),6.52...
Embodiment 3
[0047] Compound (S,E)-5-hydroxy-2-(3-hydroxy-4-methoxyphenyl)-4-oxochroman-7-yl3-(4-chlorophenyl) Preparation of acrylate (I-3).
[0048] 100 g of hesperidin and 45.4 g of potassium carbonate were dissolved in dimethylformamide at 10° C. and stirred at 90° C. for 6 hours. The reacted solution was cooled to room temperature. To the solution was added 100 g of iodomethane, followed by stirring at room temperature for 8 hours. After completion of the reaction, add 5L of ethyl acetate and dichloromethane (3:2) mixed solution therein, add nitric acid and sulfuric acid mixture after stirring, filter and collect the precipitated solid after the solution was stirred for 2 hours, and wash with a small amount of ethanol, The target compound (white solid, 39.8 g, yield: 79.6%) was obtained, and its structural formula is shown in the following formula (I-3).
[0049]
[0050] 1 H-NMR(300MHz,DMSO)δ:11.35(1H,m),8.21(2H,m),8.03(2H,m),7.62(1H,s),6.99(1H,m),6.81-6.75(2H ,J=7.5,m),6.60(...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com