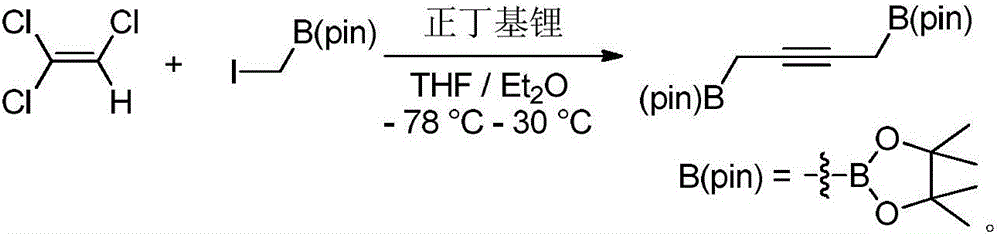
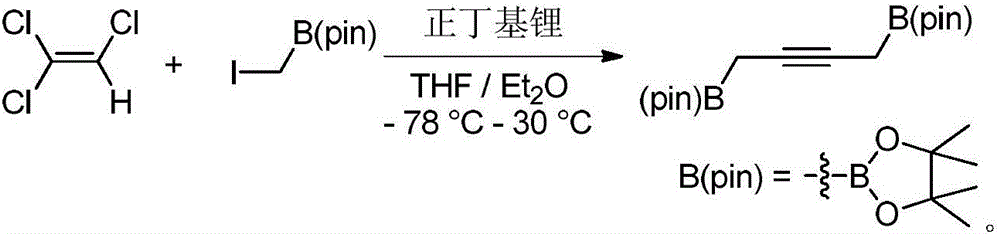
Preparation method and application of 1,4-diboric acid ester-2-butyne
A technology of diboronic acid ester and butyne, which is applied in the field of organic compound synthesis and application, and achieves the effect of easy operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0017] The preparation method of 1,4-diboronic acid ester-2-butyne, its step is: in N 2 , Under the condition of -78°C, use n-butyllithium as the base, add trichlorethylene dropwise to the flask; after the dropwise addition, raise the temperature of the mixed solution to 30°C for 12 hours, and then cool to -78°C; then Add iodomethyleneboronic acid pinacol ester dropwise to the mixed solution, continue to react for 2 hours after the dropwise addition, and then raise the temperature to 30°C for 48 hours; after the reaction is completed, add water to quench, separate and purify to obtain the product 1,4-di Borate-2-butyne, the general reaction formula is as follows:
[0018]
[0019] The application of synthesizing 1,3-butadiene-2,3-disecondary alcohol with 1,4-diboronic acid ester-2-butyne as raw material, the steps are: in N 2 Atmosphere, 30°C or 50°C or 80°C, aromatic aldehydes (benzaldehyde, 4-methylbenzaldehyde, 4-bromobenzaldehyde), 1,4-diboronate-2-butyne, toluene or ...
Embodiment 1
[0023] Preparation of 1,4-diboronate-2-butyne.
[0024]
[0025] Under nitrogen atmosphere, add 45mL THF / Et into a 250mL two-necked flask 2 A mixed solvent of O (volume ratio 1:1) and 17.5 mL of diethyl ether solution (2.5 M, 43.7 mmol) of n-butyllithium were stirred and cooled to -78 °C. Dissolve 1.3mL of trichlorethylene (14.6mmol) in 10mL of anhydrous ether, slowly drop it into the above mixed solution, raise the temperature to 30°C and stir for 12 hours to obtain a white turbid solution. Under a nitrogen atmosphere, an anhydrous ether solution (50 mL) of iodomethyleneboronic acid pinacol ester (7.8 g) was cooled to -78° C., and the above white turbid liquid was slowly added dropwise into the cooled solution with a syringe. After the dropwise addition, the reaction was continued at -78°C for 2 hours, and then the temperature was slowly raised to 30°C for 48h. After the reaction was completed, 30 mL of water was added to quench the reaction and the liquids were separate...
Embodiment 2
[0027] Preparation of 1,3-butadiene-2,3-disecondary alcohol compound
[0028]
[0029] Under nitrogen atmosphere, add benzaldehyde (10.6mg, 0.1mmol), 1,4-diboronate-2-butyne (15.3mg, 0.05mmol), 2-nitrobenzoic acid (1.0mg, 10mol%) and anhydrous toluene (0.25mL), stirred and reacted at 30°C for 48 hours, and then directly separated by column chromatography to obtain the target product 1,3-butadiene-2,3-disecondary alcohol compound. Yield 51%, dr=3.8:1.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com