Application of Monobenzone to preparation of medicaments
A technology for monobenzone and medicine preparation, which is applied in the field of use of monobenzone in medicine preparation, can solve the problems such as the need for further development of monobenzone
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
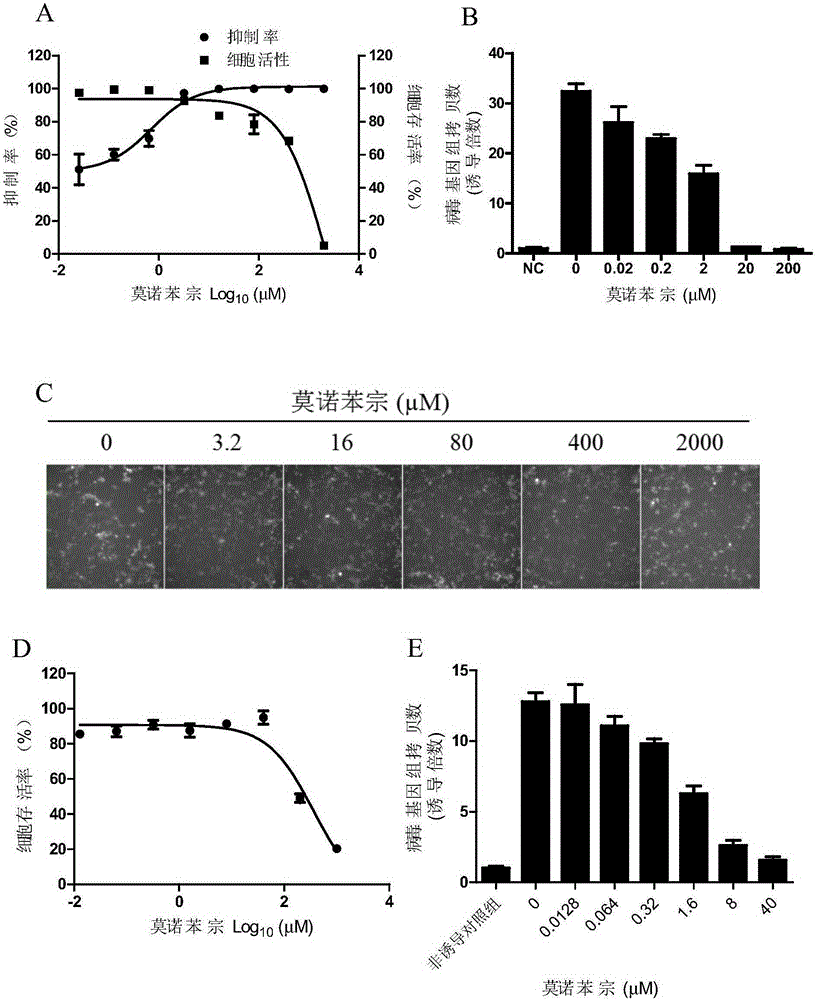
[0049] Example 1 Anti-KSHV lytic phase replication activity detection of monobenzone
[0050] 1. Experimental materials
[0051] 1.1 Cells and viruses
[0052] iSLK.219 cells were donated by Professor Dr. Don Ganem. The cells contain recombinant virus rKSHV.219 and an RTA expression system regulated by doxycycline. In this cell, the RTA expression sequence was constructed into the pRetro-XTet-ON inducible expression system (Clontech, Mountainview, CA), and the expression of this system was regulated by doxycycline. In the presence of doxycycline, RTA expression takes place. 293T cells were preserved in our laboratory and purchased from the American Type Culture Collection (ATCC).
[0053] Monobenzone was purchased from Biochempartner.
[0054] 1.2 Reagents
[0055] DMEM medium, 1640 medium and FBS were purchased from GIBCO; Alamarblue activity detection kit was purchased from Promega; SYBR mixture (iTaq TM Universal Green Supermix) was purchased from Bio-Rad Company. ...
Embodiment 2
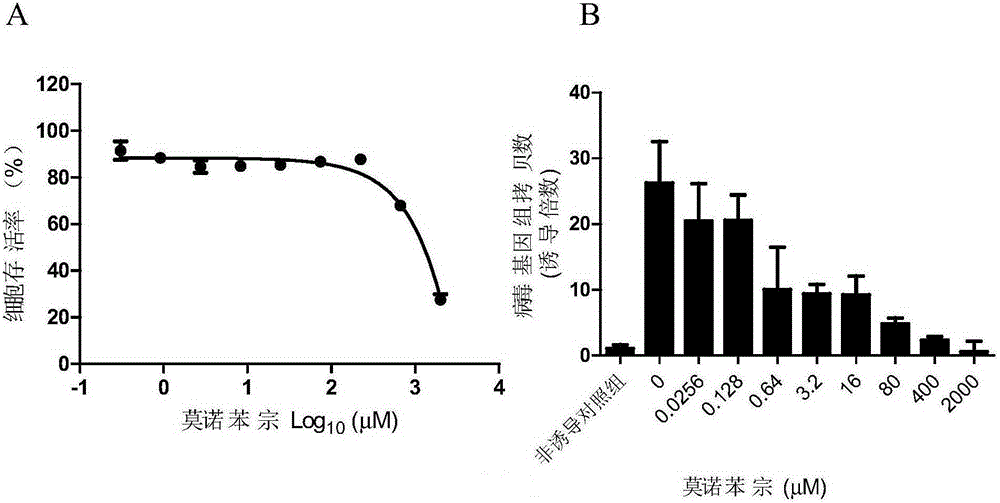
[0111] Example 2 Activity Detection of Monobenzone Anti-EBV Lysis Phase Replication
[0112] 1. Experimental materials
[0113] 1.1 Cells and viruses
[0114]HONE-1 cells were donated by Dr.George Tsao, and contain the recombinant virus BAC-EBV strain, which contains the complete sequence of the EBV genome. Co-induction with 20 ng / mL TPA and 0.3 mM sodium butyrate (NaB) can make the virus enter the lytic phase and replicate.
[0115] 1.2 Reagents
[0116] 1640 medium and FBS were purchased from GIBCO; Alamarblue activity detection kit was purchased from Promega; SYBR mixture (iTaq TM Universal Green Supermix) was purchased from Bio-Rad Company.
[0117] 1.3 Experimental Instruments
[0118] The multi-label microplate reader was purchased from PerkinElmer; the fluorescent quantitative PCR instrument (Bio-Rad CFX96 Touch TM Real-Time PCR detection system) was purchased from Bio-Rad Company; 1.0R refrigerated centrifuge and cell culture box were purchased from Thermo C...
Embodiment 3
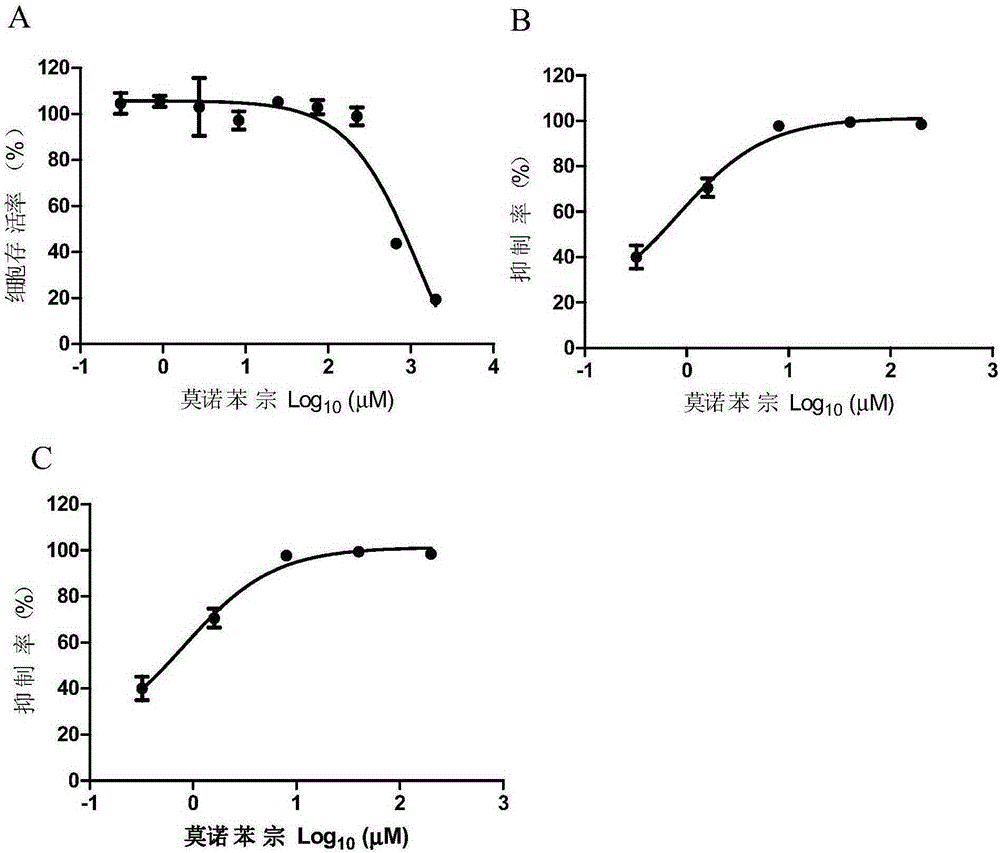
[0138] Embodiment 3: the detection of monobenzone anti-HSV-1 and HSV-2 activity
[0139] 1. Experimental materials
[0140] 1.1 Cells and viruses
[0141] Vero cells were preserved in our laboratory and purchased from the American Type Culture Collection (ATCC). HSV-1 is an F strain, which is preserved by the Wuhan Institute of Virology, Chinese Academy of Sciences. HSV-2 is a G strain, which is preserved by the Wuhan Institute of Virology, Chinese Academy of Sciences.
[0142] 1.2 Reagents
[0143] DMEM medium and FBS were purchased from GIBCO; Alamarblue activity detection kit was purchased from Promega; crystal violet staining solution.
[0144] 1.3 Experimental Instruments
[0145] The multi-label microplate reader and the high-content cell analyzer were purchased from PerkinElmer; the cell incubator was a product of Thermo.
[0146] 2. Experimental methods and results
[0147] 2.1 Cytotoxicity detection of monobenzone on Vero cells.
[0148] (1) Divide Vero cells ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com