Positron-nuclide-labeled dansyl amido diphenylethylene compound and synthesis method and application thereof
A technology based on dansylamide and methoxystilbene, which is applied in measuring devices, material analysis through optical means, instruments, etc., can solve the dual mode of PET without targeting cell apoptosis and targeting β-amyloid protein Imaging agents and other issues, to achieve the effect of easy automatic labeling, high radiochemical yield, and great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0024] Example 1 Structural identification of precursor compounds and standard compounds
[0025] 1.1 Preparation of cells, animal sources and animal models
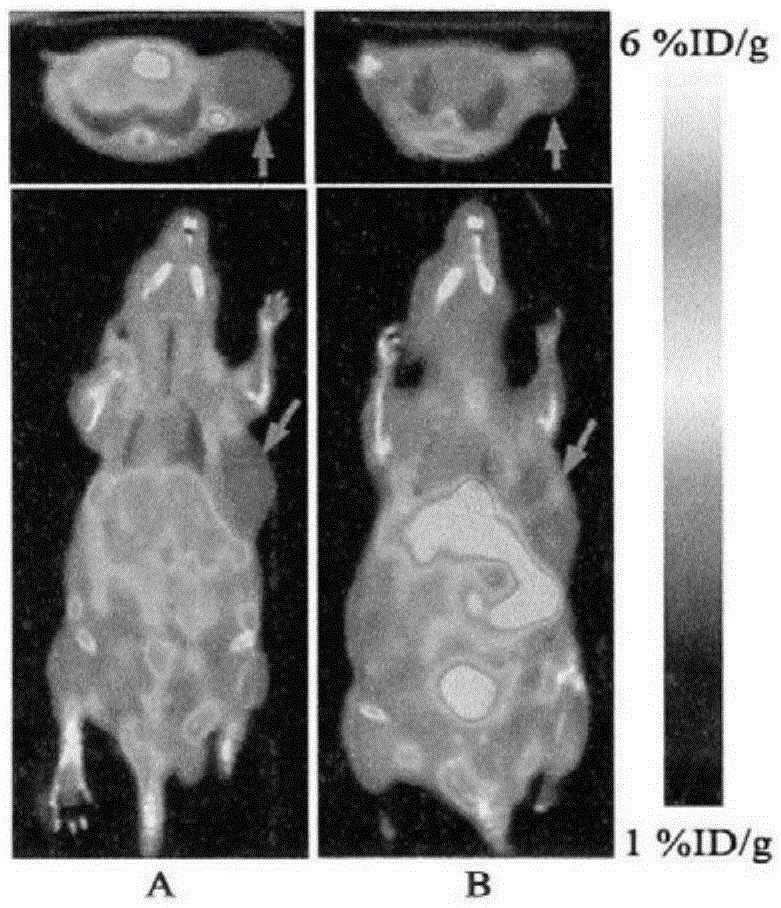
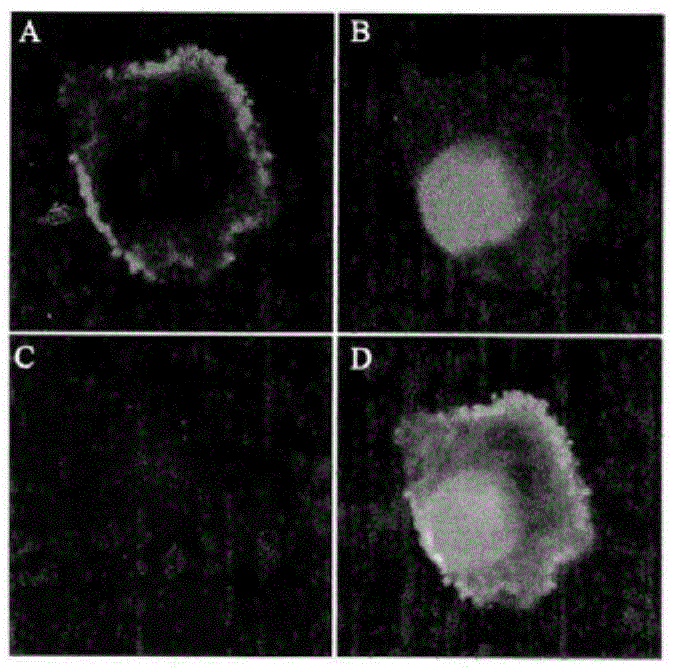
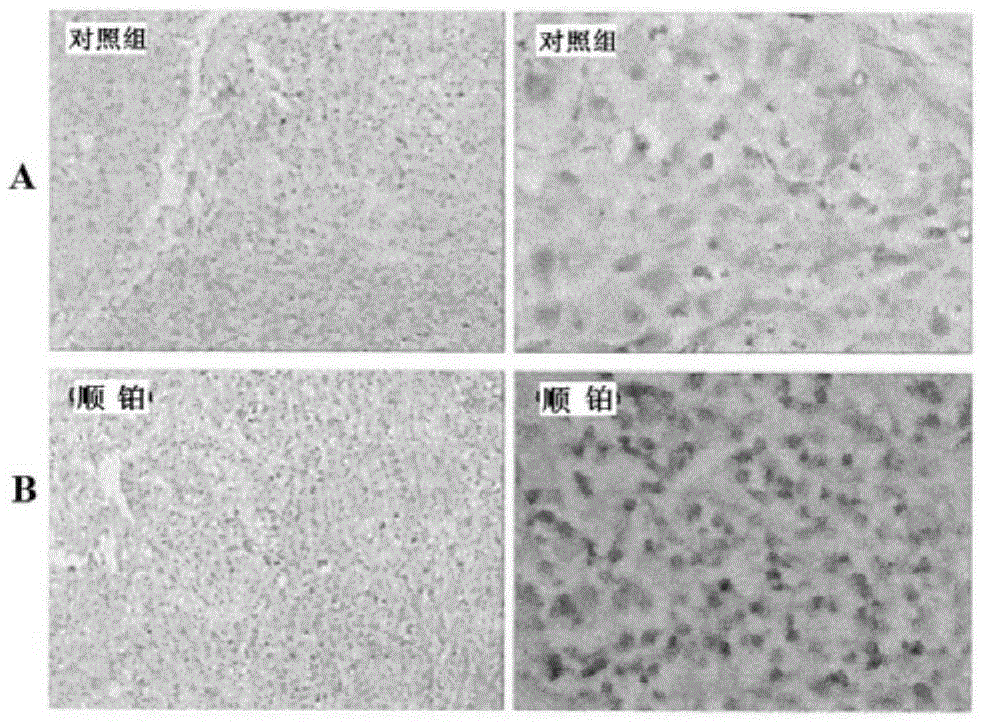
[0026] This study was approved by the Animal Experiment Ethics Committee of the First Affiliated Hospital of Sun Yat-Sen University (batch no.2012.001). S-180 fibrosarcoma cells and liver cancer cell lines were purchased from the Animal Laboratory Center of Sun Yat-sen University, Jurkat cells were purchased from the Chinese Academy of Sciences (Shanghai), and A549 cells were donated by the research group of Professor Zang Linquan from Guangzhou College of Pharmacy. Kunming mice: female, clean grade, weighing 18ˉ22g; nude mice: clean grade, four to five weeks old, purchased from the Animal Experiment Center of Sun Yat-sen University, license number SYXK (Guangdong) 2007-0081. All experimental mice were raised in the general animal laboratory of Zhongshan Medical College, 5 mice / cage, under constant temperature and humidity c...
Embodiment 211
[0040] Example 2 11 Radiosynthesis of C-DSB
[0041] Nuclear reaction by cyclotron 14 N(p,α) 11 C production 11 CO 2 , And then transferred to PET-CS-II-IT-I 11 C iodomethane synthesis module. 11 CO 2 It is trapped by a Loop ring (liquid nitrogen cooled to -160°C). Remove the liquid nitrogen cooling ring, pass in He gas (20mL / min) and release 11 CO 2 , By P 2 O 5 After the column is dried, it enters the reaction tube. Accessible 11 CO 2 With LiAlH 4 Reduction reaction occurs 11 CH 3 OH, and then react with HI to produce 11 CH 3 I. Under the action of He gas, 11 CH 3 I pass the high temperature Ag-triflate column to get 11 CH 3 -triflate( 11 C-CH 3 OTf). Before the experiment, 1 mg of the precursor 4-dansylamide-4'-hydroxystilbene (1) was dissolved in 0.2mL DMSO and 0.8mL acetone solution, 20μL 2.5M NaOH solution was added, and 0.3mL was charged into the reaction tube. 11 C-CH 3 OTf passes N 2 Carry it into the reaction tube until 11 CH 3 After I evaporated, continue to react at...
Embodiment 318
[0047] Example 3 18 Radiosynthesis of F-DFESB
[0048] Cyclotron passed 18 O(p,n) 18 F nuclear reaction produced 18 F - Ions, after being captured by QMA column, use K 222 Solution (K 2 CO 3 2.7mg dissolved in 0.1mL water, 12mg K 222 Dissolved in 0.9mL MeCN) Elution 18 F - To the reaction flask. Nitrogen gas (80 mL / min) was introduced, and the solvent was removed under heating at 116°C. Anhydrous acetonitrile (1.5 mL) was added, nitrogen gas was added and heated to 116°C, and water was removed again. Add the precursor 4-dansylamino-4'-methylsulfonyl triethoxy stilbene (3) solution (5 mg precursor dissolved in 1 mL acetonitrile) to the radioactive [K / K222] +18 F - In the reaction flask, heat to 100°C in a sealed reaction tube and keep it for 10 min. After cooling the reaction tube, add 10 mL of water to dilute and pass through the SEP PAK plus C18 column. 18 F-DFESB is adsorbed on the SEP PAK plus C18 cartridge, and the SEP PAK plus C18 cartridge is rinsed with 10 mL of water to ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com