Positron nuclide-labeled dansylamide stilbene compound, its synthesis method and application
A technology of dansylamide-based and methoxystilbenes, which is applied in the field of positronnuclide-labeled dansylamido-stilbenes, their synthesis and application, and can solve the problem of untargeted cell apoptosis and target To solve the problems of β-amyloid PET dual-mode imaging agent, achieve the effect of easy automatic labeling, high radiochemical yield, and great application prospects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] The structural identification of embodiment 1 precursor compound and standard product compound
[0021] 1.1 Preparation of cells, animal sources and animal models
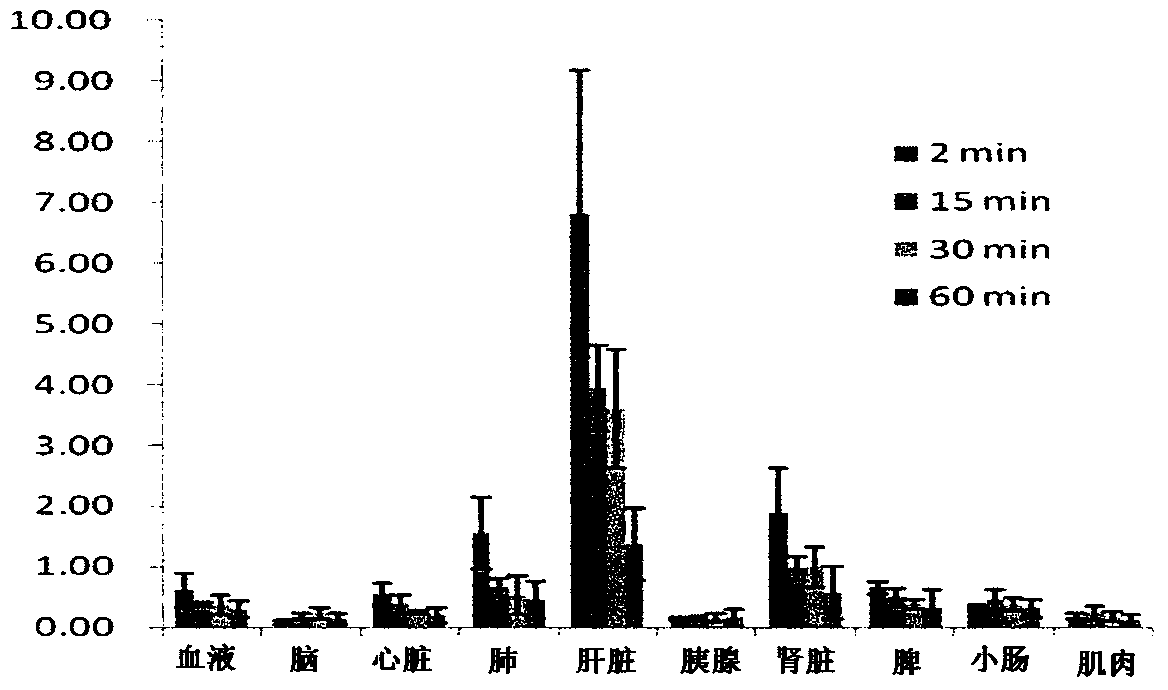
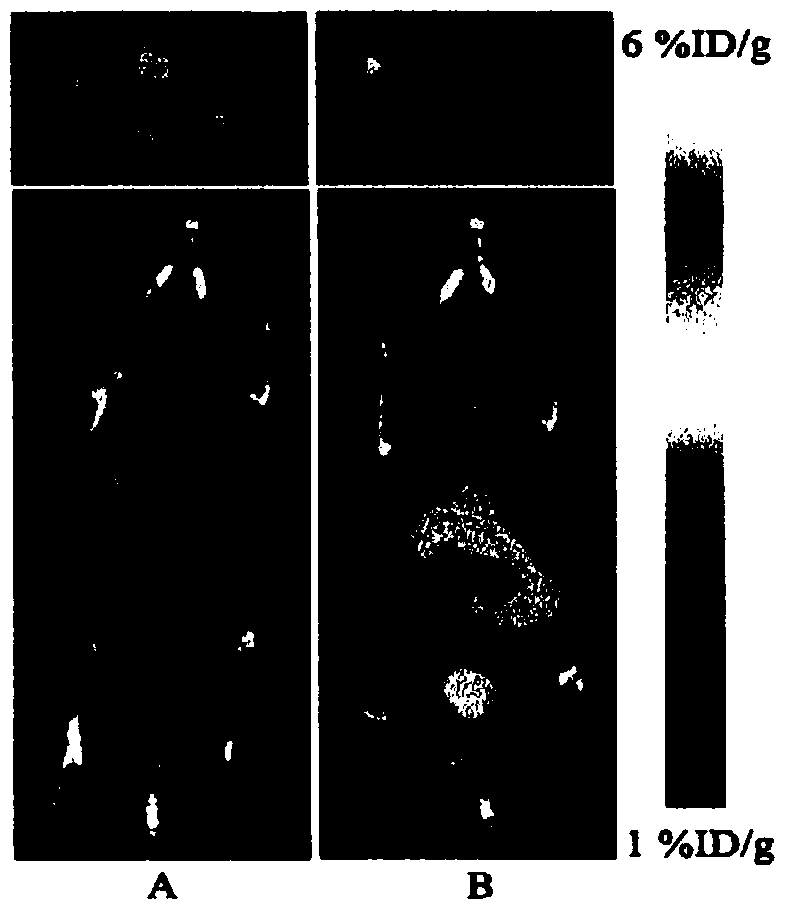
[0022] This study was approved by the Animal Experiment Ethics Committee of the First Affiliated Hospital of Sun Yat-sen University (batch number no.2012.001). S-180 fibrosarcoma cells and liver cancer cell lines were purchased from the Animal Experiment Center of Sun Yat-sen University, Jurkat cells were purchased from the Chinese Academy of Sciences (Shanghai), and A549 cells were donated by the research group of Professor Zang Linquan of Guangzhou School of Pharmacy. Kunming mice: female, clean grade, weighing 18-22 g; nude mice: clean grade, four to five weeks old, all purchased from the Animal Experiment Center of Sun Yat-sen University, license number SYXK (Guangdong) 2007-0081. All experimental mice were raised in the general-grade animal laboratory of Sun Yat-sen Medical University, 5 mice / cage, und...
Embodiment 211
[0036] Example 2 11 Radiosynthesis of C-DSB
[0037] Cyclotron via nuclear reaction 14 N(p,α) 11 C production 11 CO 2 , and then transferred to the PET-CS-II-IT-I 11 C iodide synthesis module. 11 CO 2 Trapped by the Loop (liquid nitrogen cooled to -160°C). Remove the liquid nitrogen cooling ring, pass He gas (20mL / min) and release 11 CO 2 , via P 2 o 5 After drying, the column enters the reaction tube. Accessible 11 CO 2 with LiAlH 4 A reduction reaction occurs 11 CH 3 OH, and then undergoes a substitution reaction with HI to generate 11 CH 3 I. Under the action of He gas, 11 CH 3 I passes through the Ag-triflate column of high temperature, obtains 11 CH 3 -triflate( 11 C-CH 3 OTf). Before the experiment, dissolve 1 mg of the precursor 4-dansylamido-4'-hydroxystilbene (1) in 0.2 mL of DMSO and 0.8 mL of acetone solution, add 20 μL of 2.5 M NaOH solution, and take 0.3 mL into the reaction Tube. 11 C-CH 3 OTf through N 2 carried into the reaction tu...
Embodiment 318
[0043] Example 3 18 Radiosynthesis of F-DFESB
[0044] Cyclotron through 18 O(p,n) 18 F nuclear reaction produces 18 f - Ions, after being captured by the QMA column, use K 222 Solution (K 2 CO 3 2.7mg dissolved in 0.1mL water, 12mg K 222 Dissolved in 0.9mL MeCN) eluting 18 f - to the reaction bottle. Nitrogen gas (80 mL / min) was passed, and the solvent was removed under heating at 116°C. Anhydrous acetonitrile (1.5 mL) was added, nitrogen gas was passed and heated to 116° C., and water was removed again. A solution of the precursor 4-dansylamido-4'-methanesulfonyltriethoxystilbene (3) (5 mg of the precursor dissolved in 1 mL of acetonitrile) was added to a solution containing radioactive [K / K222] +18 f - In a reaction vial, heat to 100°C in a sealed reaction tube and keep for 10 min. After cooling the reaction tube, add 10mL of water to dilute and pass through the SEP PAK plus C18 column. 18 F-DFESB was adsorbed on the SEP PAK plus C18 column, and the SEP PAK ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com