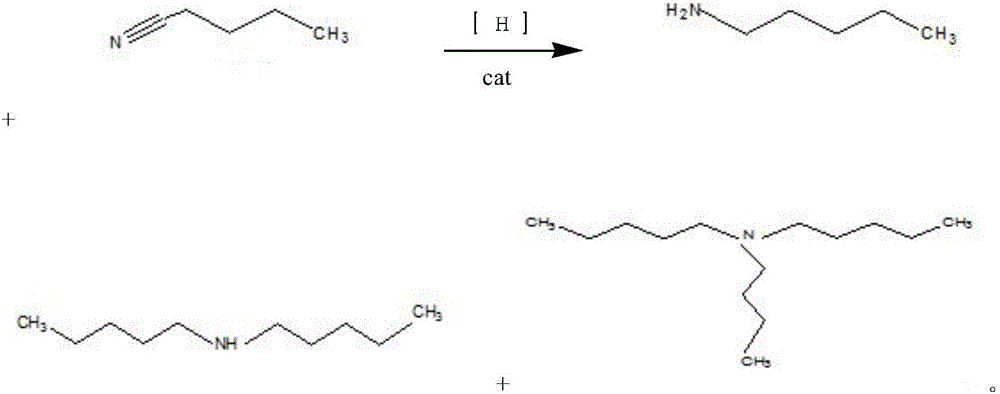
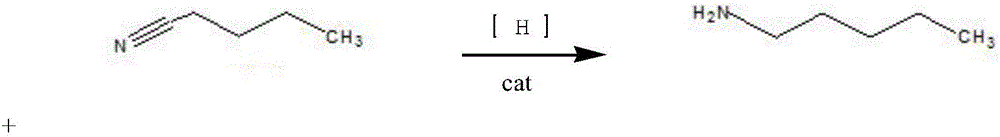
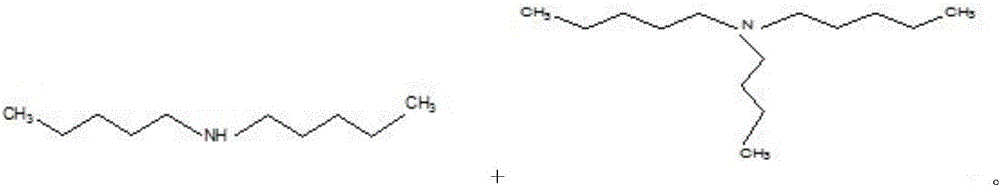
Synthesis method for nickel-base composite catalyst used for synthesizing n-amylamine from pentanenitrile
A technology of composite catalyst and synthesis method, which is applied in the field of synthesis of nickel-based composite catalyst, can solve the problems of increased cost, low yield, high cost of raw materials, etc., and achieve the effect of preventing agglomeration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1 and 2
[0022] Embodiment 1 and 2: the preparation of nickel-based composite catalyst
[0023] The catalyst carrier used in the following examples 1-6 is purchased from the market, and only the titanium-modified activated alumina carrier and the silica carrier are used as examples in the examples 1-6:
Embodiment 1
[0025] (1) Preparation of soluble nickel salt solution: Weigh 300g of nickel nitrate and put it into a 1L beaker, add 150ML deionized water and 150ML absolute ethanol, stir to dissolve; add additives EDTA 5g, diethylamine 15g, 20g of triethylamine, 5g of surfactant PEG800, 5g of PEG100005g were stirred and dissolved for later use;
[0026] (2) Preparation of the catalyst precursor: put 600 g of the purchased titanium-modified alumina carrier into the above room temperature solution and soak for 2 days and 2 nights, then take it out and ventilate and dry it in an oven at 80±1°C to obtain 700 g of the precursor for use;
[0027] (3) Catalyst precursor reduction: put 100g of the precursor into a 2L reaction kettle, put in 0.05mol / l sodium borohydride solution to cover the precursor, add a small amount of sodium hydroxide to adjust the pH to 14, start stirring and raise the temperature to 70±1℃ Down reaction, reaction is complete after 2 hours;
[0028] (4) Filter the reduced cat...
Embodiment 2
[0030] (1) Preparation of soluble nickel salt solution: Weigh 300g of nickel nitrate and put it into a 1L beaker, add 150ML deionized water and 150ML absolute ethanol, stir to dissolve; add additives EDTA 5g, diethylamine 15g, 20g of triethylamine, 5g of surfactant PEG800, 5g of PEG100005g were stirred and dissolved for later use;
[0031] (2) Preparation of catalyst precursor: Put 600g of the purchased large-pore silica carrier (particle size 1-3mm) into the above room temperature solution for 2 days and 2 nights, take it out and ventilate and dry it in an oven at 80±1°C Get 700g of precursor for later use;
[0032] (3) Catalyst precursor reduction: put 100g of the precursor into a 2L reaction kettle, put in 0.05mol / l sodium borohydride solution to cover the precursor, add a small amount of sodium hydroxide to adjust the pH to 14, and start stirring to raise the temperature to 70±1°C Down reaction, reaction is complete after 2 hours;
[0033] (4) Filter the reduced catalyst...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com