Iridium-based bipyridine-organosilicon nanotube heterogeneous catalyst and preparation method
A technology based on bipyridine and organosilicon, which is applied in the field of iridium-based bipyridine-organosilicon nanotube heterogeneous molecular catalysts and its preparation, can solve the problems of poor stability of homogeneous molecular catalysts, difficulty in recycling, and restrictions on industrial applications, and achieve easy Recovery can be recycled, good stability, low requirements for equipment and equipment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
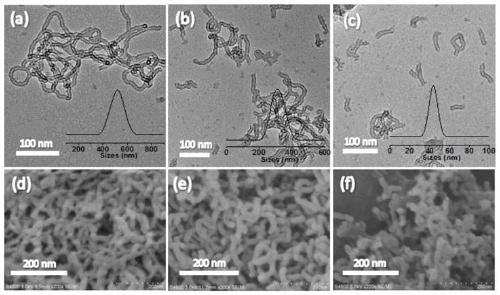
Image
Examples
Embodiment 1
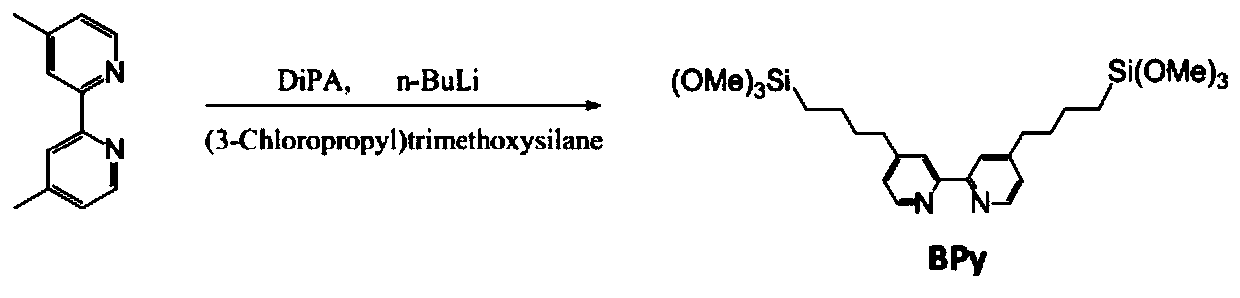
[0032] Step 1: Add 2.952g of diisopropylamine and 80mL of tetrahydrofuran into a 250mL beaker at -10°C under anhydrous nitrogen atmosphere, then add 1.74g of n-butyllithium dropwise and stir well. Afterwards, 1.21g of 4,4'-dimethyl-2,2'-bipyridine was added dropwise and stirred, and then 2.62g of 3-chloropropyltrimethoxysilane was slowly added and stirred for 12h, and the above mixture was reacted overnight Then add acetone to quench, finally evaporate the solvent, and obtain 4,4'-[4-(trimethoxysilane)butyl]-2,2'-bipyridine after vacuum drying. Synthesis process see figure 1 .
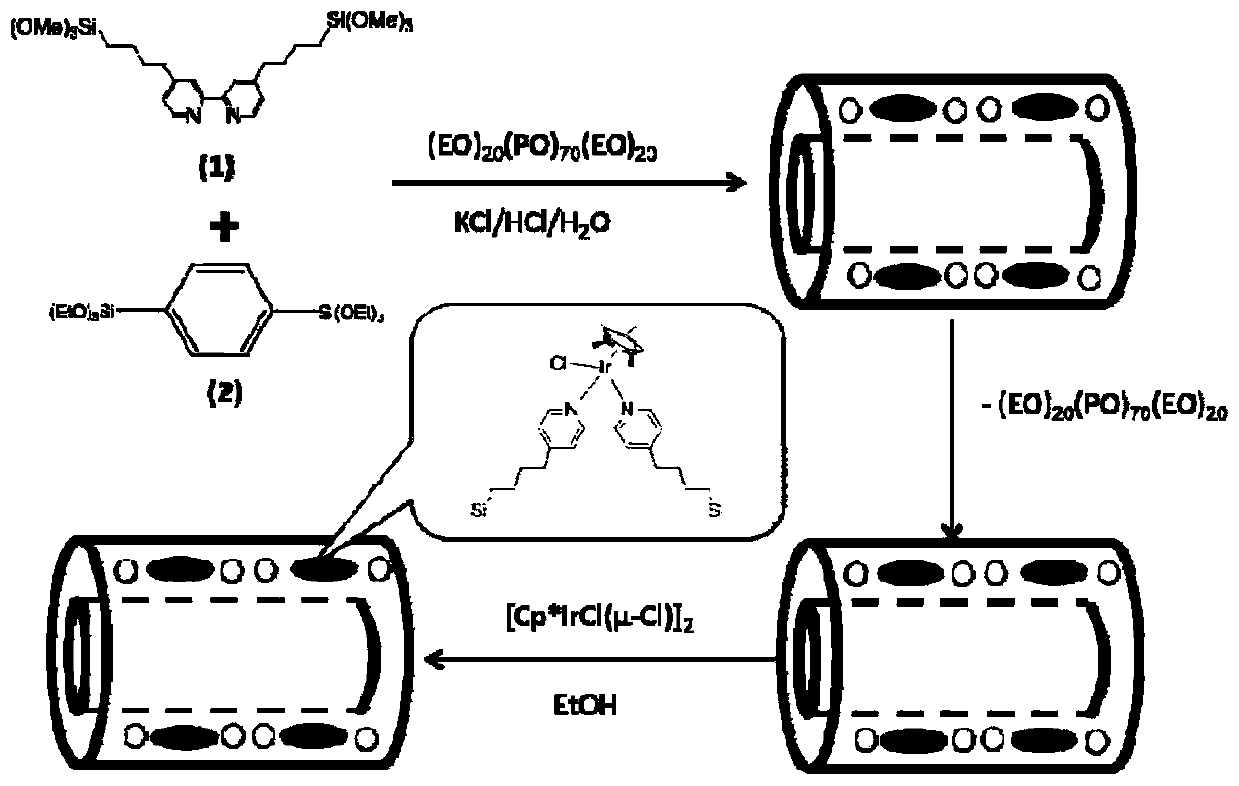
[0033] Step 2: Mix 0.875g KCl and 0.275g triblock copolymer surfactant polyoxyethylene-polyoxypropylene-polyoxyethylene EO 20 -PO 70 -EO 20 (P123) was dissolved in 120mL 2M hydrochloric acid solution, stirred rapidly to form a uniform transparent solution, added 3.15mmol organosilane precursor 1,4‐bis(triethoxysilyl)benzene and continued to stir vigorously, reducing the stirring rate Continue to s...
Embodiment 2
[0038] Step 1: Add 1.476g of diisopropylamine and 80mL of tetrahydrofuran into a 250mL beaker at -5°C under anhydrous nitrogen atmosphere, then add 0.87g of n-butyllithium dropwise and stir well. Afterwards, 1.21g of 4,4'-dimethyl-2,2'-bipyridine was added dropwise and stirred, and then 3.93g of 3-chloropropyltrimethoxysilane was slowly added and stirred for 18h, and the above mixture was reacted overnight Then add acetone to quench, finally evaporate the solvent, and obtain 4,4'-[4-(trimethoxysilane)butyl]-2,2'-bipyridine after vacuum drying. Synthesis process see figure 1 .
[0039] Step 2: Mix 1.75g KCl and 0.55g triblock copolymer surfactant polyoxyethylene-polyoxypropylene-polyoxyethylene EO 20 -PO 70 -EO 20 (P123) was dissolved in 120mL of 1.8M hydrochloric acid solution, stirred rapidly to form a uniform and transparent solution, added 2.8mmol organosilane precursor 1,4-bis(triethoxysilyl)benzene and continued to stir vigorously, reduce the stirring Continue stir...
Embodiment 3
[0044] Step 1: Add 0.738g of diisopropylamine and 80mL of tetrahydrofuran into a 250mL beaker at 0°C under anhydrous nitrogen atmosphere, then add 0.435g of n-butyllithium dropwise and stir well. After that, 1.21g of 4,4'-dimethyl-2,2'-bipyridine was added dropwise and stirred, and then 5.24g of 3-chloropropyltrimethoxysilane was slowly added and stirred for 24h, and the above mixture was reacted overnight Then add acetone to quench, finally evaporate the solvent, and obtain 4,4'-[4-(trimethoxysilane)butyl]-2,2'-bipyridine after vacuum drying. Synthesis process see figure 1 .
[0045] Step 2: Mix 3.5KCl and 1.1g triblock copolymer surfactant polyoxyethylene-polyoxypropylene-polyoxyethylene EO 20 -PO 70 -EO 20 (P123) was dissolved in 120mL 2.2M hydrochloric acid solution, stirred rapidly to form a uniform transparent solution, added 2.45mmol organosilane precursor 1,4-bis(triethoxysilyl)benzene and continued to stir vigorously, reduce the stirring Continue stirring at a hi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com