Synthesis method and application of hapten of tetrabromobisphenol A derivative TBBPA-MBPE
A technology of tetrabromobisphenol and synthesis method, which is applied in the field of surgery, can solve the problems of specificity and polarity of the analyte, and achieve the effect of strong specificity and high antibody titer
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
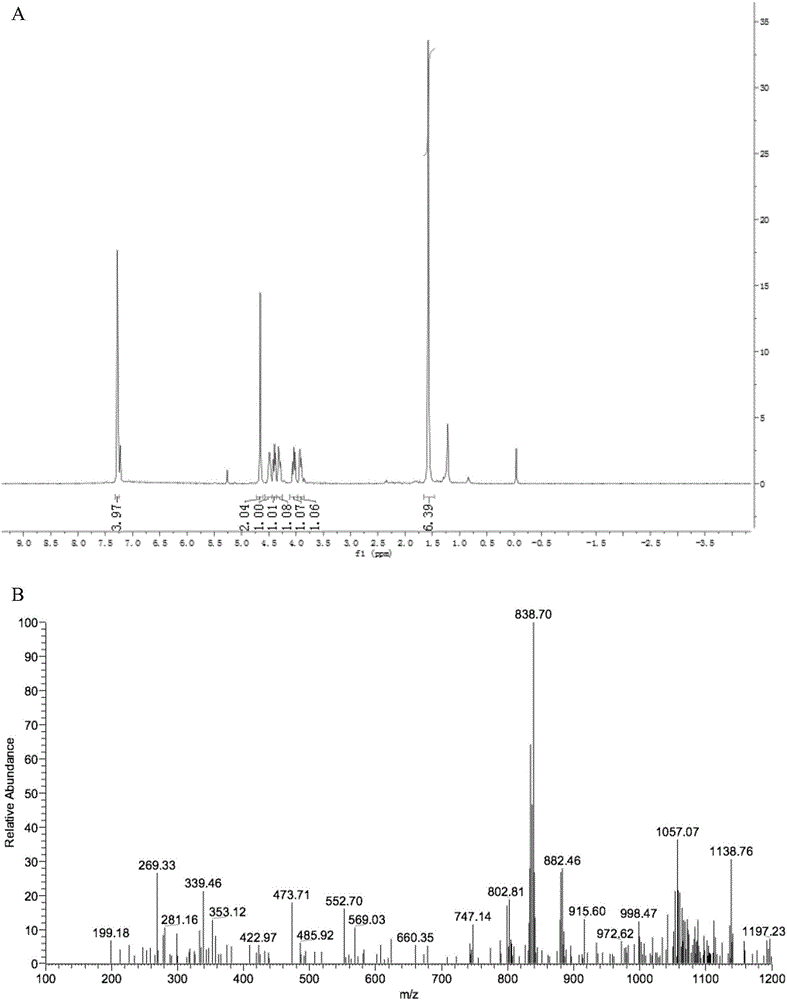
[0027] In order to make the objectives, technical solutions, and advantages of the present invention clearer, the following further describes the present invention in detail with reference to the accompanying drawings and embodiments. It should be understood that the specific embodiments described here are only used to explain the present invention, but not to limit the present invention.
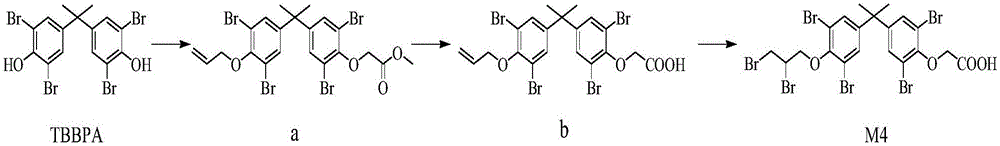
[0028] (1) Using TBBPA as a raw material, substituted with methyl bromoacetate and allyl bromide to generate methyl-2-(4-(2-(4-(allyloxy)-3,5-dibromophenyl)propane -2-yl)-2,6-dibromophenoxy)ethyl acetate (a);
[0029] Add K to 10ml of DMF with TBBPA (3.24g) dissolved 2 CO 3 (2.4g), methyl bromoacetate (918mg) was added dropwise at 100°C, and stirring was continued for 12h. Allyl bromide (0.242g, 0.002mol) was added dropwise to the reaction solution for 4h. It was washed with water, and the organic phase was extracted 3 times with 20 mL of ethyl acetate, concentrated and purified to obtain comp...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com