Heavy turpentine longifolene derivative, preparation thereof and application
A kind of technology of alkene derivatives and derivatives, applied in the field of preparation of longleaf alkene derivatives
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
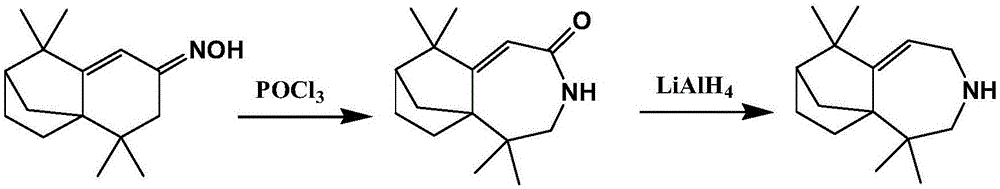
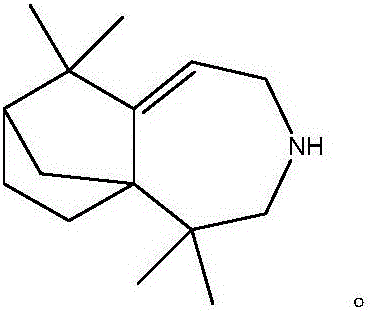
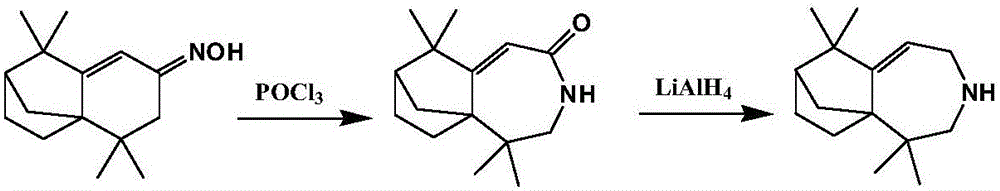
[0024] Using isolongifolenone oxime as raw material, the Beckmann rearrangement reaction is carried out to prepare isolongifolactam, and then using isolongifolactam as raw material, the longifolene derivative can be obtained through reduction reaction, namely 2, 2,8,8-Tetramethyl-4-azatricyclo[5.4.0.1 1,9 ] Dodec-6-ene. Concrete reaction equation is as follows:
[0025]
[0026] Beckmann rearrangement reaction: the present invention uses isolongifolenone oxime as raw material to prepare the isolongifolactam through Beckmann rearrangement and reduction reaction. Further specifically: isolongifolenone oxime is added to the solvent (dichloromethane) for dissolution, and then the acid-binding agent (Et 3 N) and catalyst (POCl 3 ), the mol ratio of isolongifolenone oxime, solvent, acid-binding agent, and catalyst is: 1:10:3:2, and carrying out Beckmann rearrangement reaction can obtain described isolongifolactam under inert conditions . further, which can then be sequential...
Embodiment 2
[0029] Using isolongifolenone oxime as raw material, the Beckmann rearrangement reaction is carried out to prepare isolongifolactam, and then using isolongifolactam as raw material, the longifolene derivative can be obtained through reduction reaction, namely 2, 2,8,8-Tetramethyl-4-azatricyclo[5.4.0.1 1,9 ] Dodec-6-ene. Concrete reaction equation is as follows:
[0030]
[0031] Beckmann rearrangement reaction: the present invention uses isolongifolenone oxime as raw material to prepare the isolongifolactam through Beckmann rearrangement and reduction reaction. Further specifically: isolongifolenone oxime is added to the solvent (dichloromethane) for dissolution, and then the acid-binding agent (Et 3 N) and catalyst (POCl 3 ), the mol ratio of isolongifolenone oxime, solvent, acid-binding agent, and catalyst is: 1:13:4:2.5, and carrying out Beckmann rearrangement reaction can obtain described isolongifolactam under inert conditions . further, which can then be sequenti...
Embodiment 3
[0034] Using isolongifolenone oxime as raw material, the Beckmann rearrangement reaction is carried out to prepare isolongifolactam, and then using isolongifolactam as raw material, the longifolene derivative can be obtained through reduction reaction, namely 2, 2,8,8-Tetramethyl-4-azatricyclo[5.4.0.1 1,9 ] Dodec-6-ene. Concrete reaction equation is as follows:
[0035]
[0036] Beckmann rearrangement reaction: the present invention uses isolongifolenone oxime as raw material to prepare the isolongifolactam through Beckmann rearrangement and reduction reaction. Further specifically: isolongifolenone oxime is added to the solvent (dichloromethane) for dissolution, and then the acid-binding agent (Et 3 N) and catalyst (POCl 3 ), the mol ratio of isolongifolenone oxime, solvent, acid-binding agent, and catalyst is: 1:15:5:3, and carrying out Beckmann rearrangement reaction can obtain described isolongifolactam under inert conditions . further, which can then be sequentially...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com