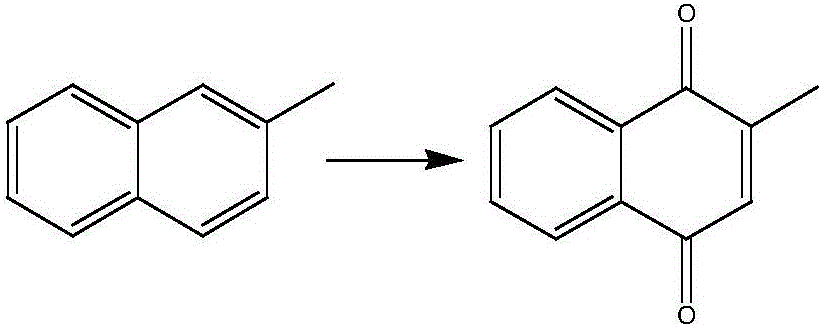
Rhenium catalyst and method for catalyzed synthesis of 2-methyl-1,4-naphthoquinone by rhenium catalyst
A catalyst and methyl technology, applied in the field of rhenium catalyst and its catalytic synthesis of 2-methyl-1,4-naphthoquinone, can solve the problems of environmental pollution, large amount of catalyst, low reaction efficiency, etc., and achieve high activity and preparation Simple method and good stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment example 1
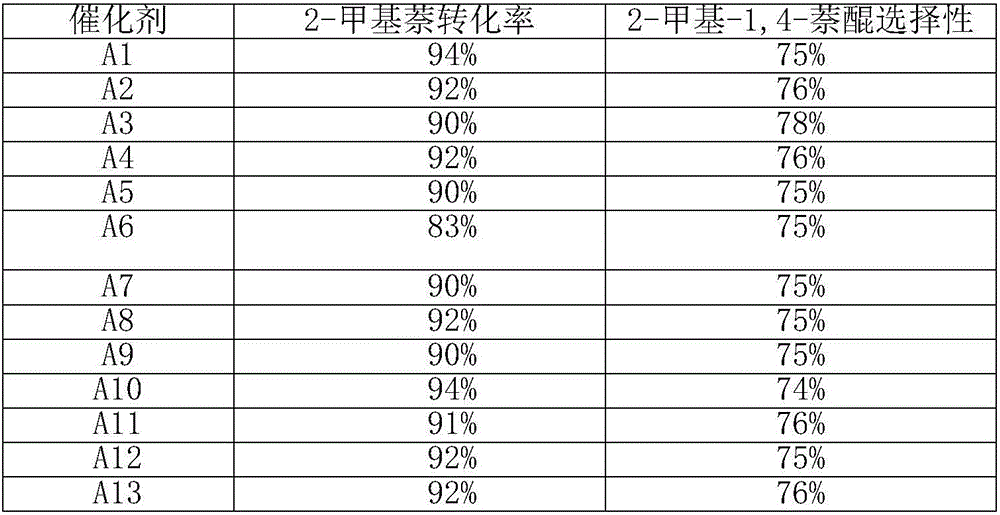
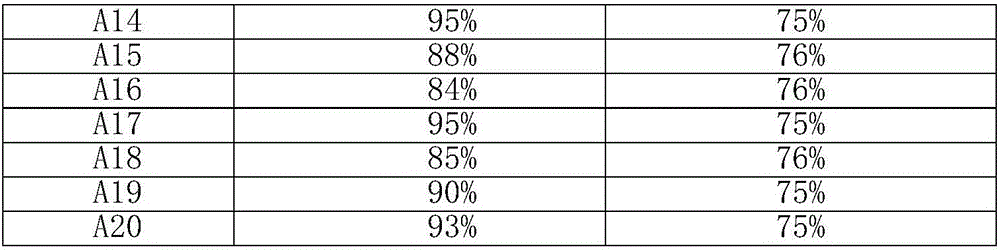
[0019] Measure the perrhenic acid solution containing 0.0100g metal rhenium in a round bottom flask, dilute it with 20ml methanol as a solvent, then add 1.0000g activated carbon to the solution, and stir at room temperature for 4h. Move the round bottom flask to an oil bath at 50°C, and add 0.1576mg of 1-methyl-3-cyanomethylimidazolium chloride into the round bottom flask, and continue stirring at this temperature for 12h. After the stirring, the round-bottomed flask was placed on a rotary evaporator to dry the solvent, and then dried overnight at 40°C in a vacuum oven, and the obtained precatalyst was calcined in a tube furnace under a nitrogen atmosphere until the temperature was 600°C. The heating rate was 5°C / min, and the temperature was maintained for 2h. Catalyst A1 (rhenium loading 1%) was obtained after natural cooling to room temperature.
Embodiment example 2
[0021] Measure the perrhenic acid solution containing 0.0100g metal rhenium in a round bottom flask, dilute it with 20ml methanol as a solvent, then add 1.0000g activated carbon to the solution, and stir at room temperature for 4h. Move the round bottom flask to an oil bath at 50°C, and add 0.1576mg of 1-methyl-3-cyanomethylimidazolium chloride into the round bottom flask, and continue stirring at this temperature for 12h. After the stirring, the round-bottomed flask was placed on a rotary evaporator to dry the solvent, then dried overnight at 40°C in a vacuum oven, and the obtained precatalyst was calcined in a tube furnace under a nitrogen atmosphere until the temperature was 700°C. The heating rate was 5°C / min, and the temperature was maintained for 2h. Catalyst A2 (rhenium loading 1%) was obtained after natural cooling to room temperature.
Embodiment example 3
[0023] Measure the perrhenic acid solution containing 0.0100g metal rhenium in a round bottom flask, dilute it with 20ml methanol as a solvent, then add 1.0000g activated carbon to the solution, and stir at room temperature for 4h. Move the round bottom flask to an oil bath at 50°C, and add 0.1576mg of 1-methyl-3-cyanomethylimidazolium chloride into the round bottom flask, and continue stirring at this temperature for 12h. After the stirring, the round-bottomed flask was placed on a rotary evaporator to dry the solvent, and then dried overnight at 40°C in a vacuum oven, and the obtained precatalyst was calcined in a tube furnace under a nitrogen atmosphere until the temperature was 800°C. The heating rate was 5°C / min, and the temperature was maintained for 2h. After naturally cooling down to room temperature, catalyst A3 (rhenium loading was 1%) was obtained.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com