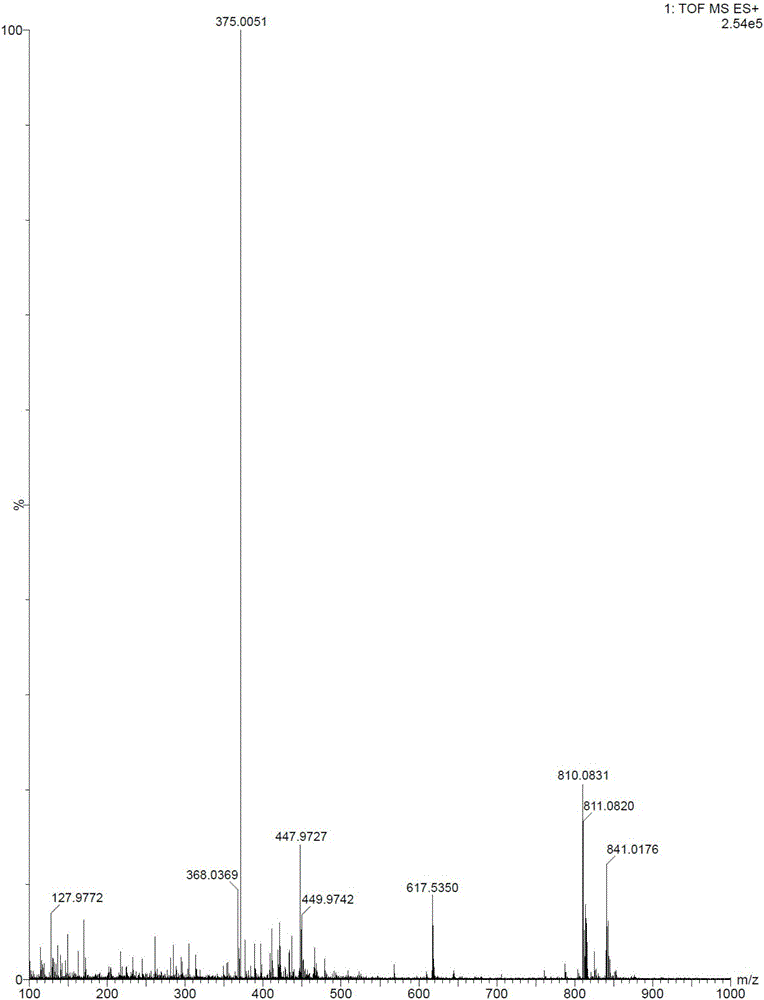Preparation method of bromhexine hydrochloride
A technology of bromhexine hydrochloride and bromhexine hydrochloride, applied in the field of pharmaceutical synthesis, can solve the problems of high requirements on reaction equipment, existence of safety risks, incomplete reaction and the like, and achieves the effects of less impurity content, easy operation and shortened reaction steps.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
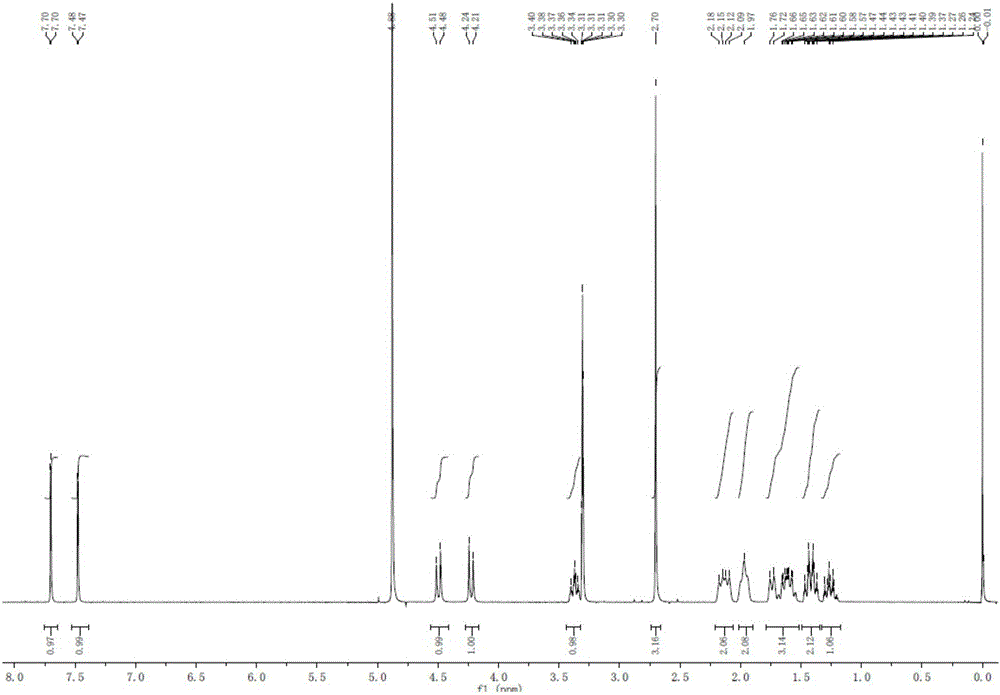
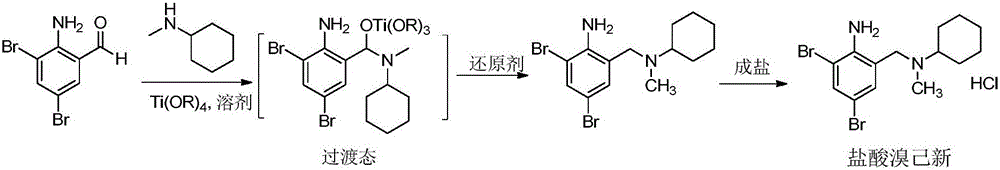
[0032] The raw materials in this example are all commercially available. Dissolve 2.0g of 2-amino-3,5-dibromobenzaldehyde and 1.2g of N-methylcyclohexylamine in 20ml of methanol, slowly drop 4g of tetraisopropyl titanate into the reaction solution while stirring at room temperature After the addition, continue to stir at room temperature for 5 h; add 0.3 g of sodium borohydride to the reaction solution in batches, and stir for 2 h at room temperature. TLC detects that the reaction is complete. After the reaction was over, add 20ml of water to the reaction solution to quench the reaction, a large amount of solids were separated out, filtered, the filter cake was washed 3 times with ethyl acetate, the filtrate and washings were combined, extracted with ethyl acetate to obtain an organic phase, which was successively washed with sodium carbonate solution , and saturated brine to wash the organic phase.
[0033] At room temperature, under stirring conditions, 5ml of 6N hydrochlo...
Embodiment 2
[0035] The raw materials in this example are all commercially available. Dissolve 2.0g of 2-amino-3,5-dibromobenzaldehyde and 4g of N-methylcyclohexylamine in 20ml of methanol, slowly drop 2g of tetraisopropyl titanate into the reaction solution while stirring at 10°C After the addition, continue to stir and react at 10° C. for 5 h; add 0.3 g of sodium borohydride to the reaction liquid in batches, and stir and react at room temperature for 2 h. TLC detects that the reaction is complete. After the reaction was over, add 20ml of water to the reaction solution to quench the reaction, a large amount of solids were separated out, filtered, the filter cake was washed 3 times with ethyl acetate, the filtrate and washings were combined, extracted with ethyl acetate to obtain an organic phase, which was successively washed with sodium carbonate solution , and saturated brine to wash the organic phase.
[0036] Under the condition of stirring at 10°C, 10ml of 6N hydrochloric acid was...
Embodiment 3
[0038] The raw materials in this example are all commercially available. Dissolve 2.0g of 2-amino-3,5-dibromobenzaldehyde and 1.2g of N-methylcyclohexylamine in 20ml of isopropanol, and slowly drop 4g of tetraisopropyl titanate under stirring at 30°C Added to the reaction solution, after the addition, continued to stir at room temperature for 5 h; added 0.3 g of sodium borohydride to the reaction solution in batches, and stirred at 30° C. for 2 h. TLC detects that the reaction is complete. After the reaction was over, add 20ml of water to the reaction solution to quench the reaction, a large amount of solids were separated out, filtered, the filter cake was washed 3 times with ethyl acetate, the filtrate and washings were combined, extracted with ethyl acetate to obtain an organic phase, which was successively washed with sodium carbonate solution , and saturated brine to wash the organic phase.
[0039] Under the condition of stirring at 30°C, 5ml of 6N hydrochloric acid wa...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com