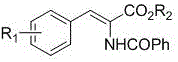
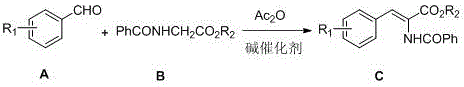Method for synthesizing 2-benzamido-3-aryl acrylate
A technology of aryl acrylate and benzoyl, which is applied in the field of synthesis of 2-benzamido-3-aryl acrylate, can solve the problems of long reaction time, complicated operation, low yield, etc., and achieve shortening Effects of reaction time, increased yield, ease of handling and control
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
example 1
[0021] Under the protection of argon, 0.1 mol (10.6 g) of benzaldehyde, 0.1 mol (19.2 g) of methyl hippurate, 0.1 mol (24.6 g) of cesium trifluoroacetate, and 100 mol of acetic anhydride were successively added to a dry 500 mL three-necked flask. mL, control the reaction temperature at 100°C, stir the reaction for 8 h, and detect that the reaction is complete, add 250 mL of water and stir to quench the reaction, extract twice with 100 mL of dichloromethane, separate and combine the dichloromethane layer, and use 100 mL ×2 washed with water, dried overnight with 15 g of anhydrous magnesium sulfate, filtered, recovered the solvent under reduced pressure to obtain a tan solid, and recrystallized from methanol to obtain 24.3 g of an off-white solid with a purity of 98.4% and a yield of 86.4%. 1 H NMR (400 MHz, DMSO- d 6 ) δ : 3.74(s, 3H), 7.35~7.44(m, 4H), 7.52~7.63(m,3H), 7.69(d, J =7.2Hz, 2H), 8.00(d, J =7.2Hz, 2H), 10.14(s, 1H). 13 C NMR (100MHz, DMSO- d 6 ) δ : 166.2, ...
example 2
[0023] Under the protection of argon, 0.105 mol (19.4 g) of 4-bromobenzaldehyde, 0.1 mol (19.2 g) of methyl hippurate, 0.1 mol (8.2 g) of sodium acetate, and 0.1 mol (8.2 g) of sodium acetate were added successively to a dry 500 mL three-necked flask 100 mL, control the reaction temperature at 110 °C, stir the reaction for 7 h, and detect the completion of the reaction in the liquid phase, add 250 mL of water and stir to quench the reaction, extract twice with 100 mL of dichloromethane, separate and combine the dichloromethane layer, and use 100 mL×2 washed with water, dried overnight with 15 g of anhydrous magnesium sulfate, filtered, and the solvent was recovered under reduced pressure to obtain a tan solid, which was recrystallized from methanol to obtain 30.2 g of an off-white solid with a purity of 98.6% and a yield of 83.8%. 1 H NMR (400 MHz, DMSO- d 6 ) δ : 3.75(s, 3H), 7.41(s, 1H), 7.53~7.63(m, 7H), 8.00(d, J =7.6Hz, 2H), 10.16(s, 1H). 13 C NMR (100MHz, DMSO- d 6...
example 3
[0025] Under argon protection, 0.1 mol (15.1 g) of p-nitrobenzaldehyde, 0.1 mol (19.2 g) of methyl hippurate, 0.1 mol (8.2 g) of sodium acetate, and acetic anhydride 105 mL, control the reaction temperature at 95 ° C, stir the reaction for 5.5 h, the liquid phase detection reaction is complete, add 250 mL of water and stir to quench the reaction, extract twice with 100 mL of dichloromethane, separate and combine the dichloromethane layer, and use 100 mL×2 washed with water, dried overnight with 15 g of anhydrous magnesium sulfate, filtered, and the solvent was recovered under reduced pressure to obtain a tan solid, which was recrystallized from methanol to obtain 27.6 g of an off-white solid with a purity of 98.4% and a yield of 84.6%. 1 H NMR (400 MHz, DMSO- d 6 ) δ : 3.77(s, 3H), 7.45(s, 1H), 7.55(t, J =7.6Hz,2H), 7.63(t, J =7.4Hz, 1H), 7.90(d, J =8.8Hz, 2H), 7.98(d, J =7.6Hz, 2H), 8.26(d, J =8.8Hz, 2H), 10.31(s, 1H). 13 C NMR (100MHz, DMSO- d 6 ) δ : 166.3, 165.2...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com