A kind of polyalkyne amine compound and preparation method thereof
A compound, polyalkyne amine technology, applied in the field of polyalkyne amine compounds and the preparation of the polyalkyne amine compounds through the reaction of alkyne halides and sulfonamides, achieving the effects of strong tolerance, energy saving, and good application value
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] A kind of polyalkyne amine compound P1, its structural formula is as follows:
[0037]
[0038] The polyalkyne amine compound is prepared by reacting an alkyne halide with a sulfonamide monomer, and its specific reaction equation is as formula (1):
[0039]
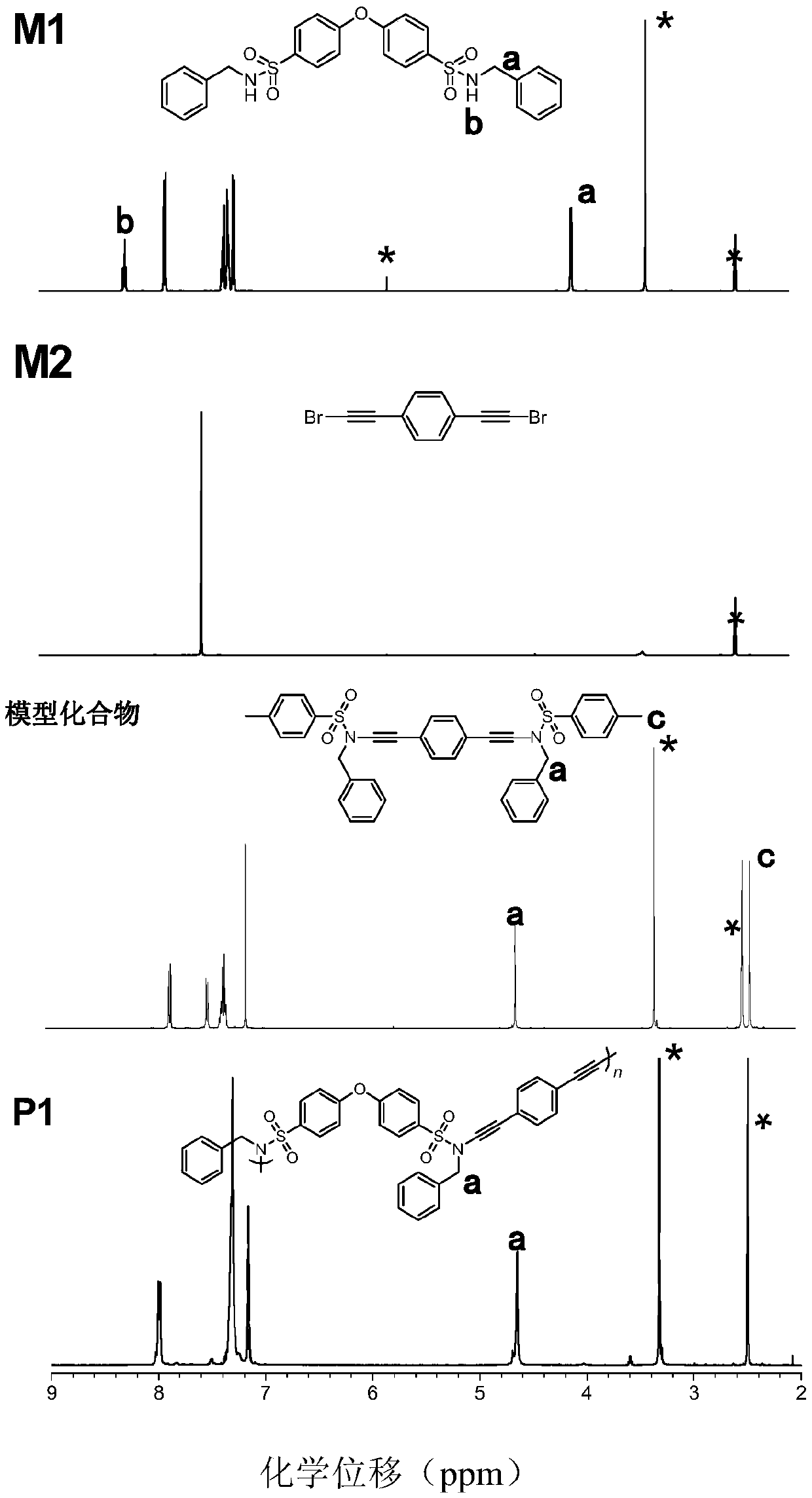
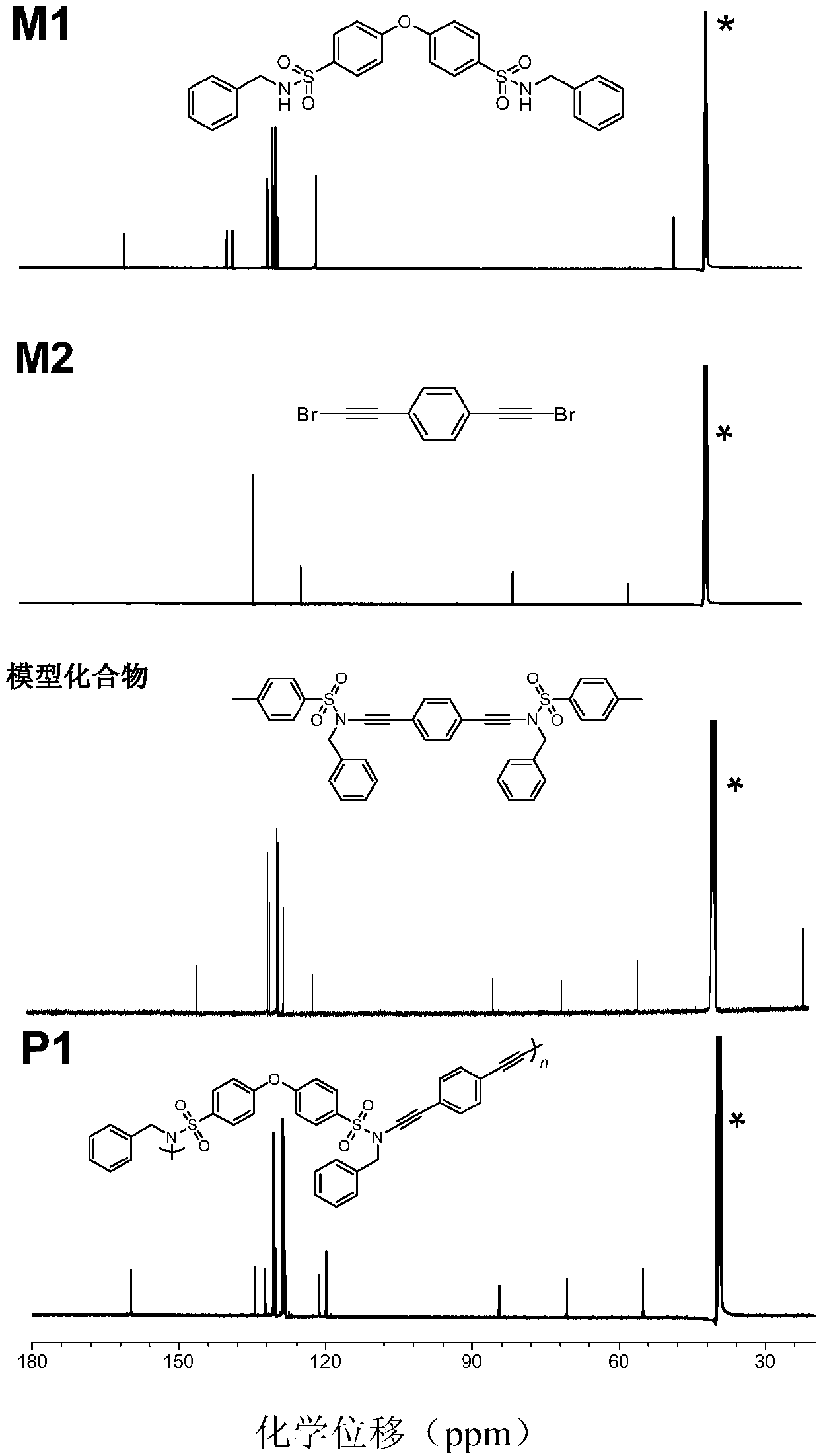
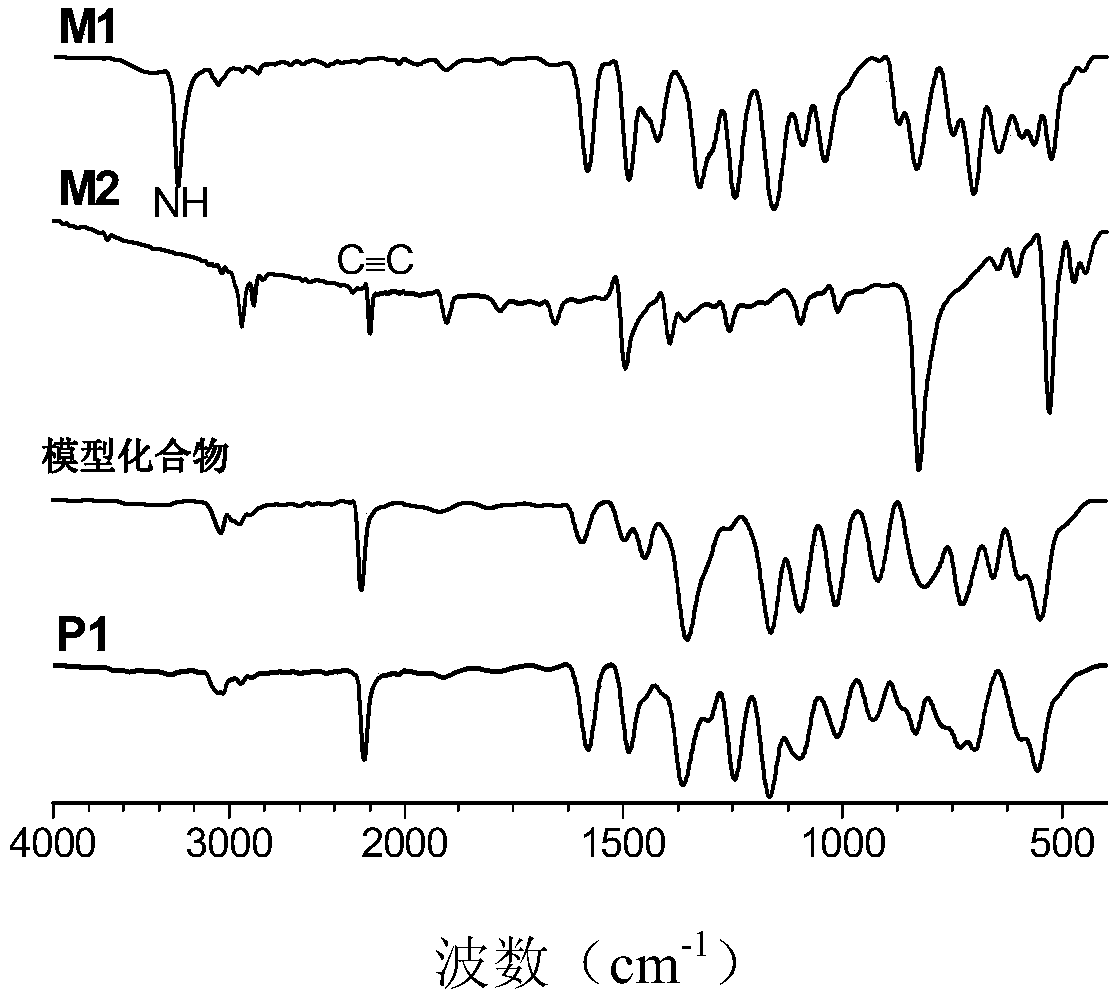
[0040] (1) The synthesis method of monomer M1 is: at 0°C, add 9.0mmol (3.29g) of sulfonyl chloride into a 250mL two-necked bottle, vacuumize and replace N 2 After adding 150mL of dichloromethane and 36.9mmol (4.03mL) benzylamine, the reaction system was stirred at room temperature for 2 hours, extracted three times with dichloromethane and water, spin-dried, and the crude product was separated and purified by silica gel chromatography Vacuum drying at 40°C gave 7.7 mmol (3.90 g) of product (monomer M1) with a yield of 85%; figure 1 8.20 of the M1 monomer in the NMR diagram is -NH peak, and 4.04 is -CH 2 Peak, wherein, 3.32 is water peak, and 2.50 is DMSO-d6 peak; image 3 There is a -NH peak in monomer M1;...
Embodiment 2
[0048] In this example, the reaction time in Example 1 was changed to 80° C., and other preparation conditions were the same as in Example 1 to obtain 0.13 g of polymer with a yield of 90% and a molecular weight of 17000 g / mol.
Embodiment 3
[0050] In this embodiment, the CuSO in embodiment 1 4 ·5H 2 O feed intake is changed into 0.04mmol (0.007g), 1,10-phenanthroline feed intake is changed into 0.08mmol (0.02g), K 2 CO 3 The feeding amount was changed to 0.80 mmol (0.11 g), and the other preparation conditions were the same as in Example 1 to obtain 0.12 g of polymer with a yield of 81% and a molecular weight of 18000 g / mol.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com