Solid-state freeze-dried product of quantitative strain and preparation method and using method thereof
A dry and solid-state technology, which is applied in the field of preparing test strains that meet the requirements of the biological inspection method in the Chinese Pharmacopoeia, can solve the problems of waste of resources and tediousness, and achieve the effects of improving accuracy, making the preparation fast and simple, and not easy to break
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
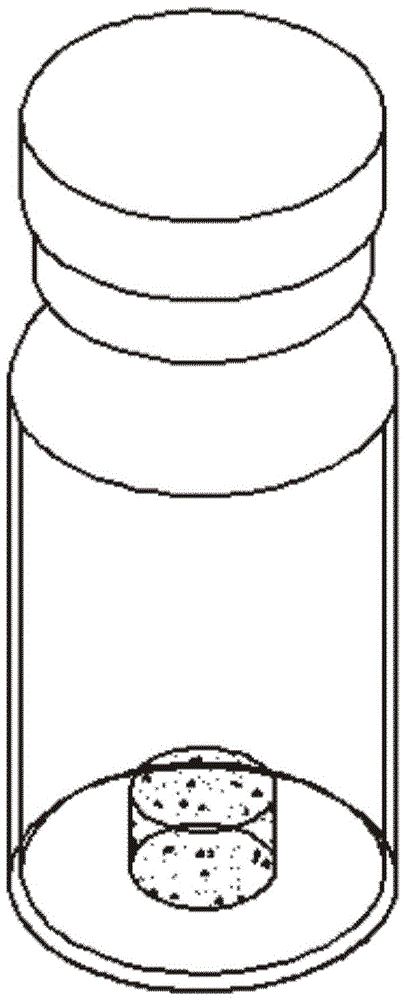
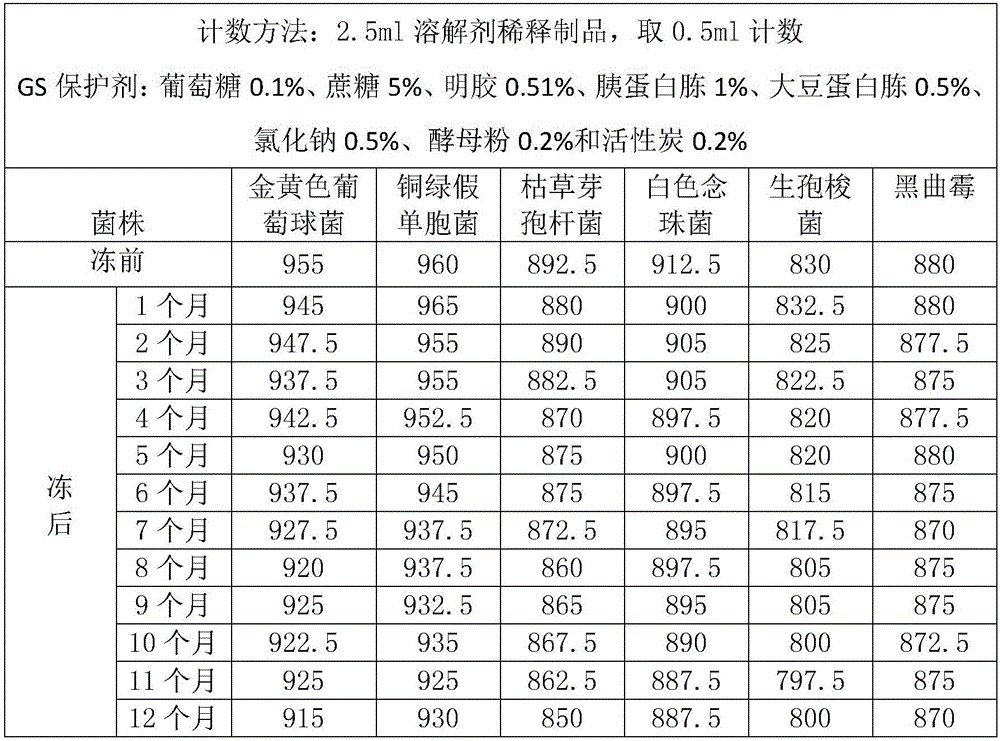
[0033] See Figure 1-2 In this case, a solid, freeze-dried product of a quantitative strain, including glucose 0.1%-0.5%, sucrose 5%-10%, gelatin 0.5%-1.5%, tryptone 1%-2%, soy peptone 0.5%- 1%, sodium chloride 0.5%-1%, yeast powder 0.2%-0.5% and activated carbon 0.2%-0.5% are formulated into a protective agent composed of a solution. The protective agent and the bacteria liquid are uniformly mixed in the required proportion and then frozen It is dried into a cylindrical dried product, and the cylindrical freeze-dried product contains 100 cfu-1000 cfu viable bacteria. Or you can customize the number of bacteria in a specific range.
[0034] Each cylindrical freeze-dried product is made from 50ul-100ul freeze-dried bacteria suspension. Of course, the volume of the specific freeze-dried bacteria suspension can be customized according to the needs of the cylindrical freeze-dried product.
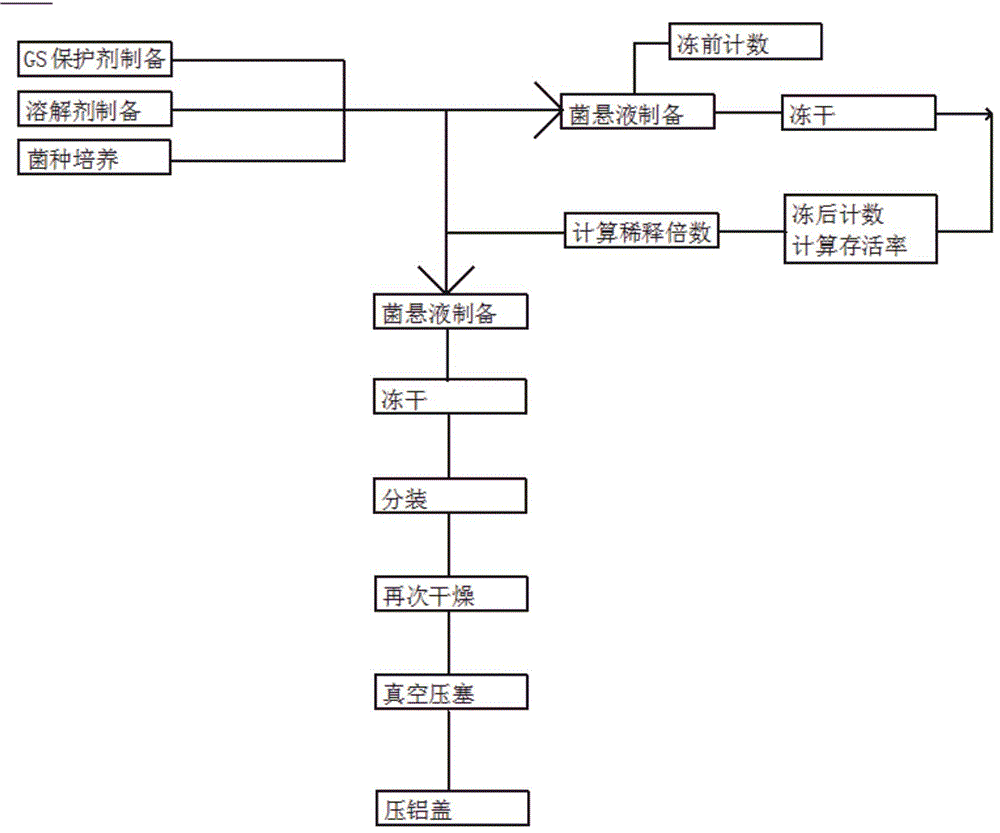
[0035] A method for preparing solid and freeze-dried products of quantitative strains includes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com