Porcine Epidemic Diarrhea Virus and application thereof
A porcine epidemic diarrhea and virus technology, applied in the direction of virus, antiviral agent, virus antigen component, etc., can solve the problem of poor treatment effect, and achieve the effect of resisting attack, high antibody titer, and small immune dose
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
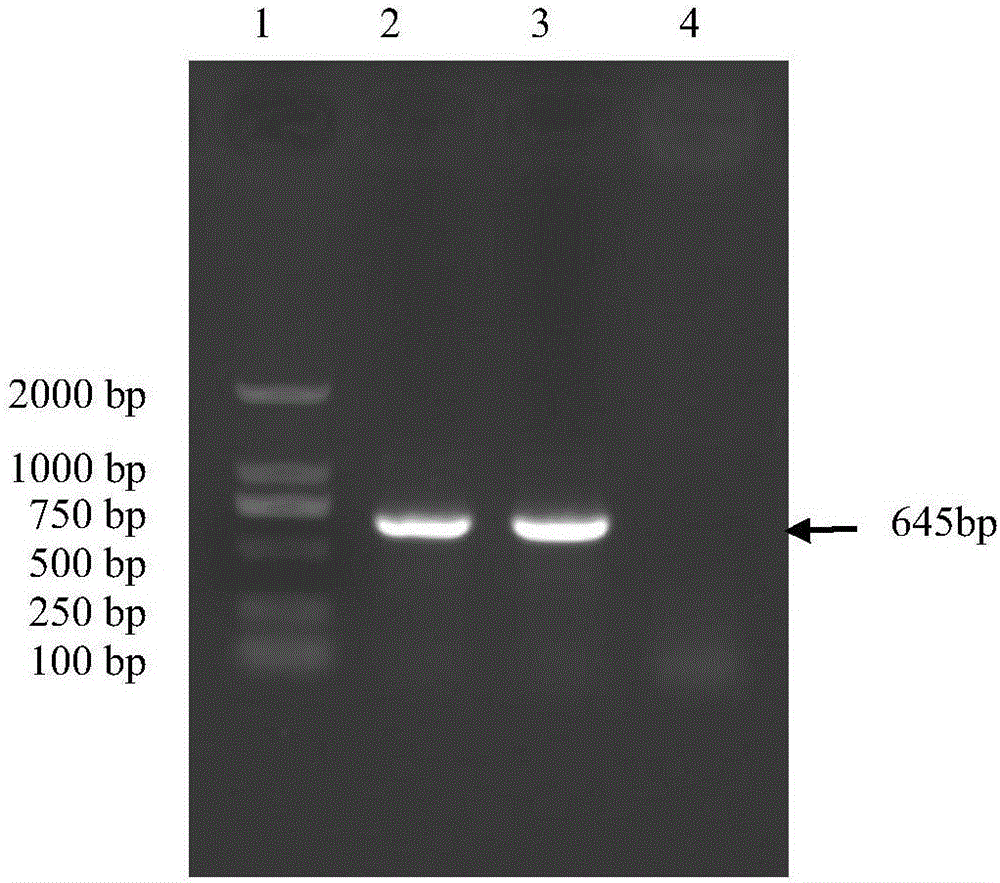
[0015] Example 1: Isolation and Identification of Porcine Epidemic Diarrhea Virus PEDV-KB2013-4 Strain
[0016] (1) Isolation of porcine epidemic diarrhea virus PEDV-KB2013-4 strain
[0017] During the epidemiological investigation, a pig farm in Shaanxi sent the small intestine of piglets with diarrhea for inspection, scraped the intestinal mucosa and contents, added PBS at a ratio of 1:5 (weight: volume), repeated freezing and thawing 3 times, and centrifuged to obtain the supernatant. Filter through a 0.22 μm filter membrane, add trypsin with a final concentration of 20 μg / ml to the filtrate, and treat at 37° C. for 1.5 hours.
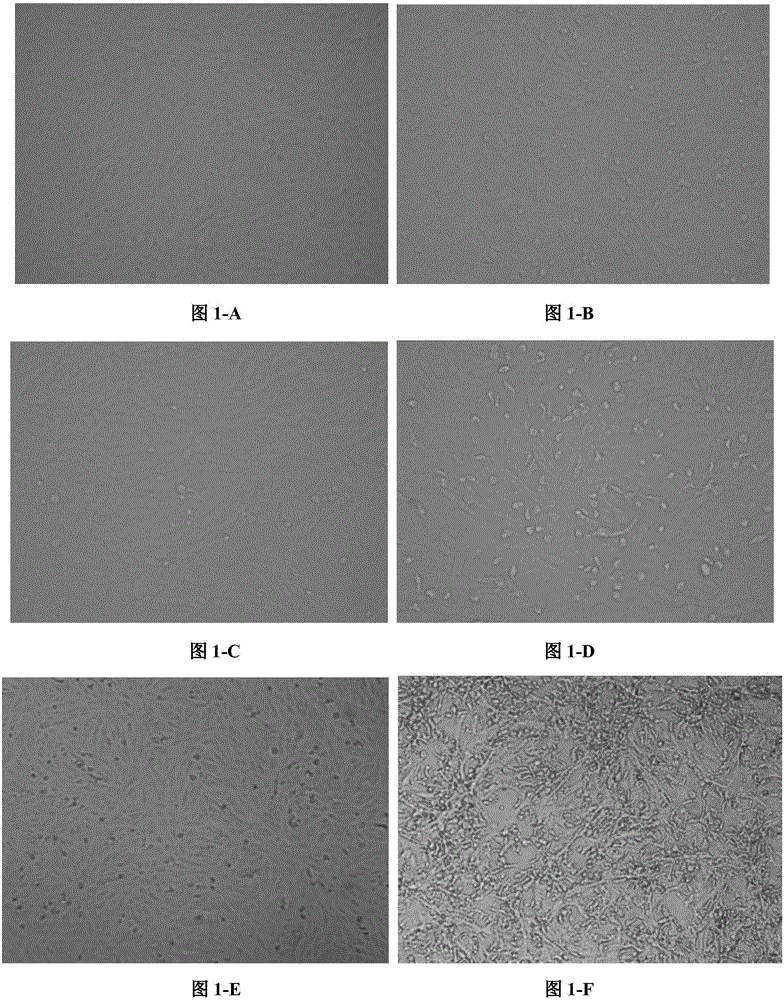
[0018] Inoculate Vero cells with a monolayer according to the conventional method (wash three times with PBS of pH 7.4 before inoculation), inoculate the virus at a ratio of 10%, absorb at 37°C for 1 hour, and supplement the cell maintenance solution (containing 10 μg / ml trypsin) ), cultivated in a 37°C incubator. In this way, the cells were blind...
Embodiment 2
[0031] Embodiment 2: Prepare vaccine with PEDV-KB2013-4 strain
[0032] (1) Preparation of PEDV vaccine
[0033] a. Prepare Vero cells according to conventional methods (Vero cells are purchased from ATCC, USA), the growth medium is DMEM containing 10% newborn bovine serum, and inoculated with PEDV-KB2013-4 virus when the cells grow into a monolayer;
[0034] b. Take the PEDV-KB2013-4 strain (5×10 5 TCID 50 / ml) inoculated well-growing Vero monolayer cells at 1%, placed at 37°C for 1 hour, discarded the adsorption solution, and added DMEM containing 10 μg / mL trypsin as the maintenance solution to continue the culture;
[0035] c. After inoculation, observe twice a day and record the cell lesions. Harvest the virus when the cell lesions reach more than 80%. Freeze and thaw the harvested virus three times at -80°C, centrifuge at 6000 rpm for 20 min, and collect the supernatant The liquid is the virus liquid;
[0036] d. After the virus liquid was concentrated 20 times, it wa...
Embodiment 3
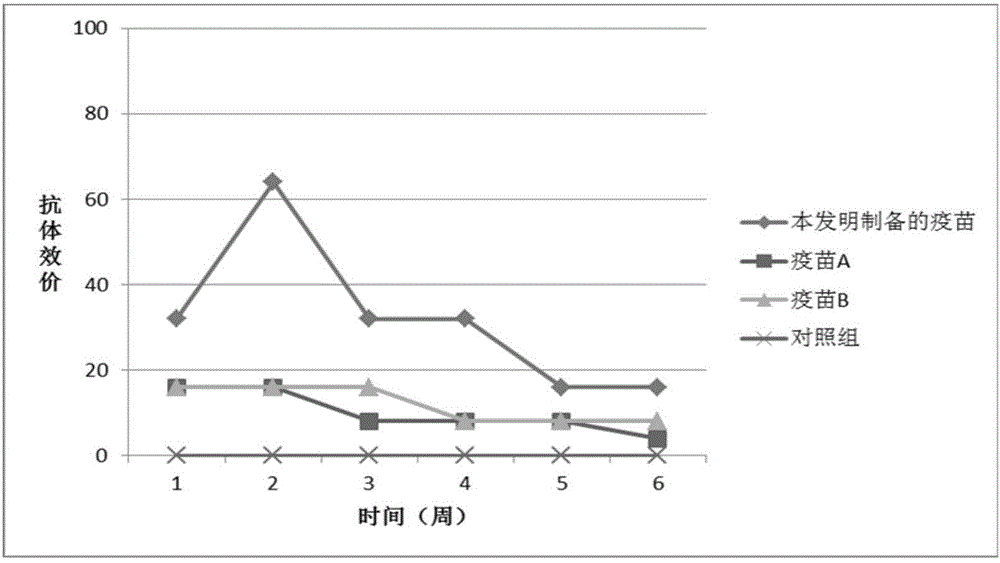
[0048] Example 3 Safety Test of Porcine Epidemic Diarrhea Virus PEDV-KB2013-4 Strain Inactivated Vaccine
[0049] 1. Materials and methods
[0050] 1.1 Single-dose test on pregnant sows
[0051] Take 10 pregnant sows that were negative for porcine epidemic diarrhea neutralizing antibodies and antigens 5-6 weeks before delivery, and randomly divided them into 2 groups with 5 pigs in each group. The first group was intramuscularly injected with 20131201 batches of porcine epidemic diarrhea virus PEDV- The KB2013-4 strain inactivated vaccine was 1 dose per head, and the second group was not injected as the control group, and the sows were observed until the sows gave birth.
[0052] 1.2 Single-dose repeated test on pregnant sows
[0053] Take 10 pregnant sows that were negative for porcine epidemic diarrhea neutralizing antibodies and antigens 5-6 weeks before delivery, and randomly divided them into 2 groups with 5 pigs in each group. The first group was intramuscularly inject...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com