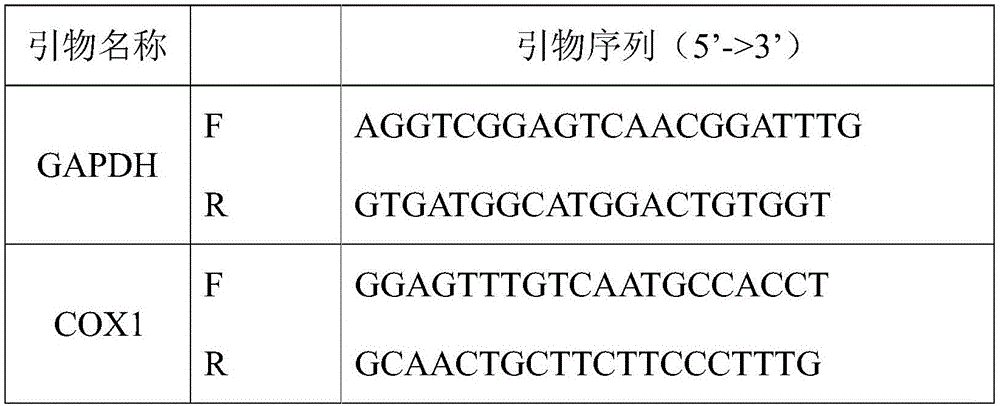
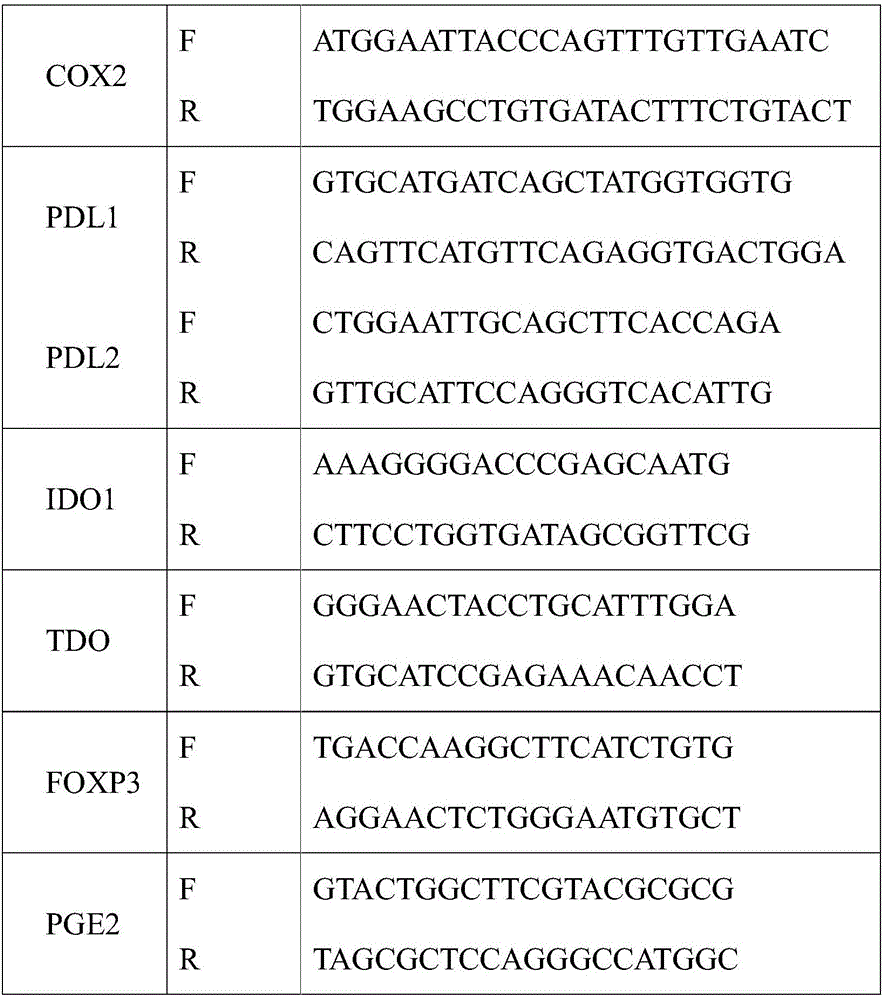
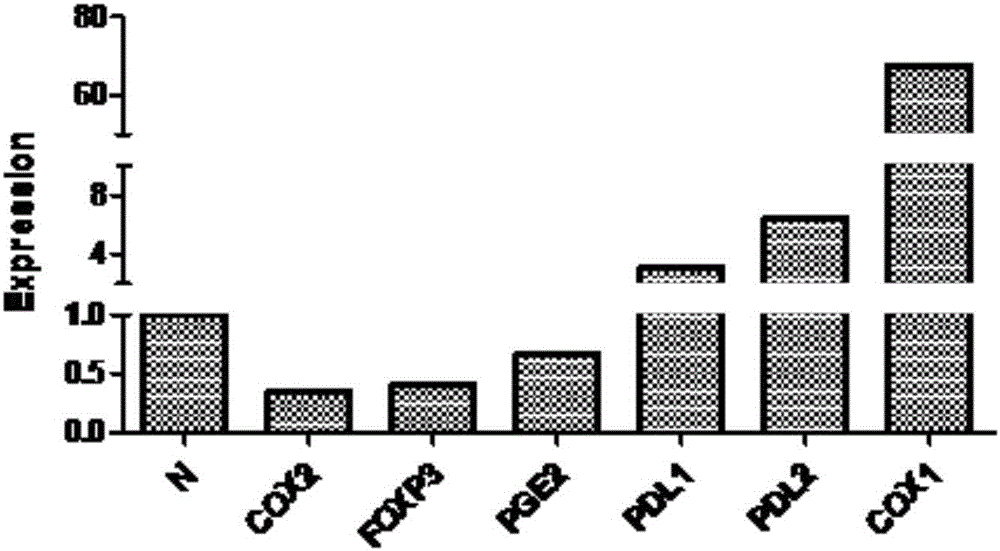Method for detecting tumor microenvironment-associated antigen expression
A tumor microenvironment and antigen technology, which is applied in biochemical equipment and methods, microbial determination/examination, DNA/RNA fragments, etc. Strong reference effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0036] Example 1: Sample RNA Extraction from Solid Tumor Tissue Samples
[0037] 1.1 Selection and preparation of tissue samples: surgically resected tumor tissue and benign tumor tissue used as a negative control, the margins were excised, and the intact tissue pieces in the middle were selected and stored in RNAlater at -80°C in the shortest possible time to prevent RNA degradation; Among them, RNAlater is a liquid, non-toxic tissue preservation reagent. It rapidly stabilizes tissues and preserves non-frozen cellular RNA in situ. After the tissue block is obtained, it is quickly soaked in RNAlater for preservation without causing RNA degradation, so that it is not necessary to process the sample immediately or freeze the sample in liquid nitrogen for later processing. It is a routine reagent in this field and can be obtained from commercial channels get.
[0038] 1.2 Take 30mg RNAlater to save the sample, add 1ml TRIzol, fully grind to lyse the tissue block, add chloroform...
Embodiment 2
[0043] Embodiment two: test sample RNA
[0044] The RNA concentration of the tissue sample was accurately measured with an ultra-micro spectrophotometer, wherein the model of the ultra-micro spectrophotometer used in this example was Nanodrop 2000. The quality analysis of RNA was carried out with a bioanalyzer, wherein the model of the bioanalyzer used in this example was Agilent 2100, and RNA samples with an OD value between 1.8 and 2.0 and a RIN value > 6 met the antigen expression profile detection standard , is a qualified RNA sample, which is used for the next experiment.
Embodiment 3
[0045] Example 3: Using the extracted sample RNA as a template and oligo dT as a primer to perform RNA reverse transcription to obtain tissue sample cDNA
[0046] Configure the cDNA acquisition reaction system shown in Table 1 on ice, mix the reaction system gently, centrifuge briefly, and then perform the procedures shown in Table 2 to obtain template cDNA.
[0047] Table 1. cDNA acquisition reaction system
[0048] components volume 10X RT Buffer (buffer) 2.0ul 25X dNTP Mix 0.8ul 10X oligo(dT) 2.0ul Reverse Transcriptase 1.0ul RNase Inhibitor 1.0ul sample RNA 1ug Nuclease-free H 2 O (nucleic acid-free pure water)
up to 20ul
[0049] Table 2. cDNA acquisition reaction program
[0050] step 1 step 2 step 3 step 4 temperature 25℃ 37℃ 85℃ 4℃ time 10min 120min 5min ∞
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com