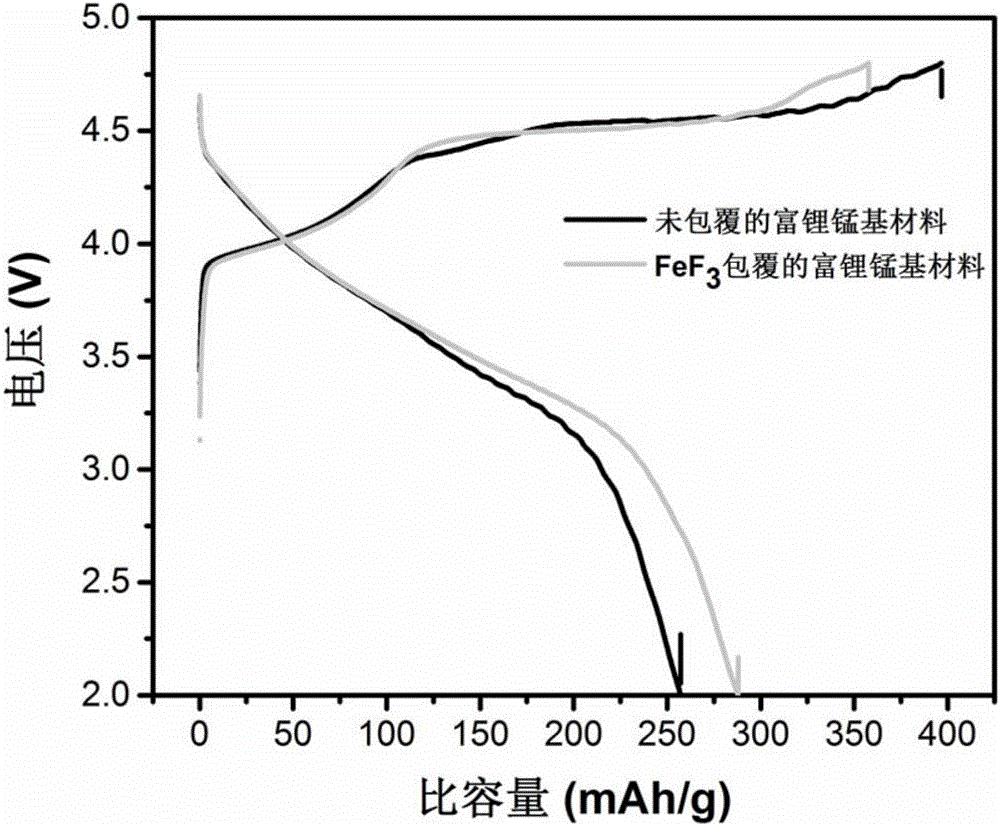
Composite positive electrode material of lithium ion battery and preparation method for composite positive electrode material
A composite positive electrode material and lithium-ion battery technology, applied in battery electrodes, secondary batteries, circuits, etc., can solve the problems that the coating layer does not have electrochemical activity and the specific capacity Coulomb efficiency decreases, so as to suppress side reactions and prevent The effect of corroding the electrode surface and avoiding direct contact
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
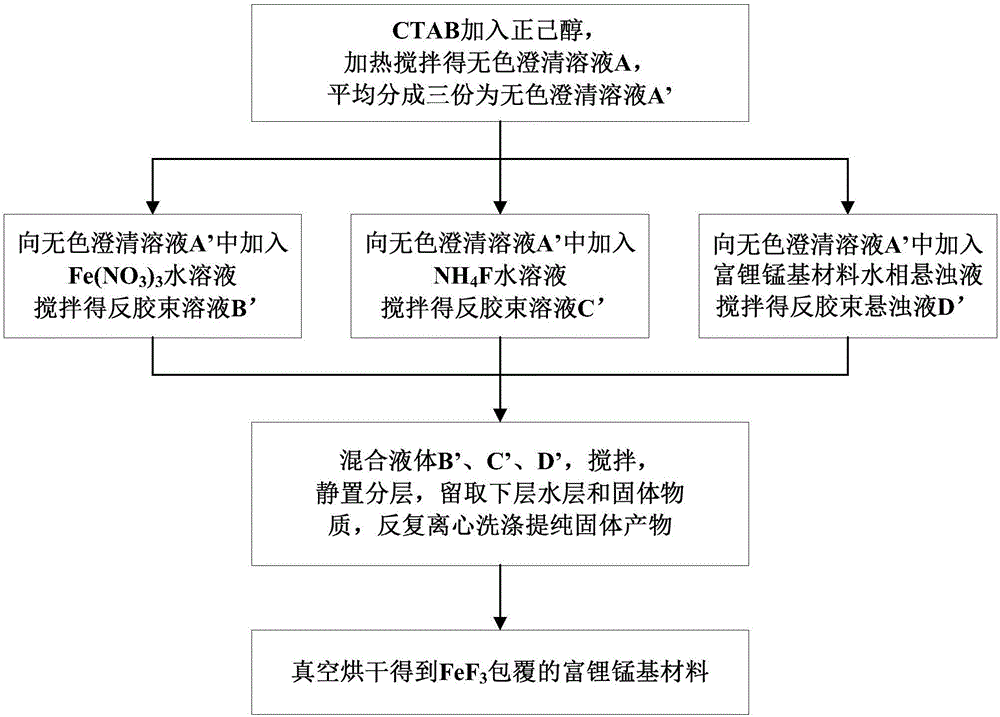
[0020] Embodiment one, such as figure 1 shown, including the following steps:
[0021] (1) 37.5g cetyltrimethylammonium bromide (CTAB) was added in 225g n-hexanol, stirred at a temperature of 40°C for 2h to obtain a colorless clear solution; (the mass ratio of CTAB to n-hexanol was 1 :6)
[0022] (2) The colorless clear solution is divided into three parts on average, and Fe(NO 3 ) 3 Aqueous solution, NH 4 The F aqueous solution and the aqueous phase suspension of lithium-rich manganese-based materials are stirred respectively to obtain two kinds of reverse micellar solutions and a kind of reverse micellar suspension; the Fe(NO 3 ) 3 Aqueous solution, NH 4 The mass ratio of the water solvent in the F aqueous solution and the aqueous phase suspension of lithium-rich manganese-based materials is equal to 12.5mL; the mass ratio of the water solvent in each aqueous solution or aqueous phase suspension to the n-hexanol in the colorless clear solution 1:6;
[0023] The Fe(NO...
Embodiment 2
[0028] Embodiment two, comprises the following steps:
[0029] (1) 225g cetyltrimethylammonium bromide (CTAB) is joined in 225g n-hexanol, stirred at 50 ℃ of temperature for 5h, obtains colorless clear solution; (the mass ratio of CTAB and n-hexanol is 1: 1)
[0030] (2) The colorless clear solution is divided into three parts on average, and Fe(NO 3 ) 3 Aqueous solution, NH 4 The F aqueous solution and the aqueous phase suspension of lithium-rich manganese-based materials are stirred respectively to obtain two kinds of reverse micellar solutions and a kind of reverse micellar suspension; the Fe(NO 3 ) 3 Aqueous solution, NH 4 The mass ratio of the water solvent in the F aqueous solution and the aqueous phase suspension of the lithium-rich manganese-based material is equal to 25mL; the mass ratio of the water solvent in each aqueous solution or aqueous phase suspension to the n-hexanol in the colorless clear solution is 1:3;
[0031] The Fe(NO 3 ) 3 and NH 4 The rati...
Embodiment 3
[0035] Embodiment three, comprises the following steps:
[0036] (1) 90g cetyltrimethylammonium bromide (CTAB) is joined in 900g n-hexanol, stirred at a temperature of 30°C for 1h to obtain a colorless clear solution; (the mass ratio of CTAB and n-hexanol is 1: 10)
[0037] (2) The colorless clear solution is divided into three parts on average, and Fe(NO 3 ) 3 Aqueous solution, NH 4 The F aqueous solution and the aqueous phase suspension of lithium-rich manganese-based materials are stirred respectively to obtain two kinds of reverse micellar solutions and a kind of reverse micellar suspension; the Fe(NO 3 ) 3 Aqueous solution, NH 4 The mass ratio of the water solvent in the F aqueous solution and the aqueous phase suspension of lithium-rich manganese-based materials is equal to 6mL; the mass ratio of the water solvent in each aqueous solution or aqueous phase suspension to the n-hexanol in the colorless clear solution is 1:50;
[0038] The Fe(NO 3 ) 3 and NH 4 The ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| current efficiency | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com