Nickel phosphide catalyst, method for preparing same and application of nickel phosphide catalyst
A catalyst, nickel phosphide technology, applied in the direction of physical/chemical process catalysts, molecular sieve catalysts, chemical instruments and methods, etc., can solve the problems of complex process, inability to realize catalyst in-situ synthesis-hydrodesulfurization reaction, etc., and achieve the preparation process Simplify the process, solve the effect of high reduction temperature and reduction promotion
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
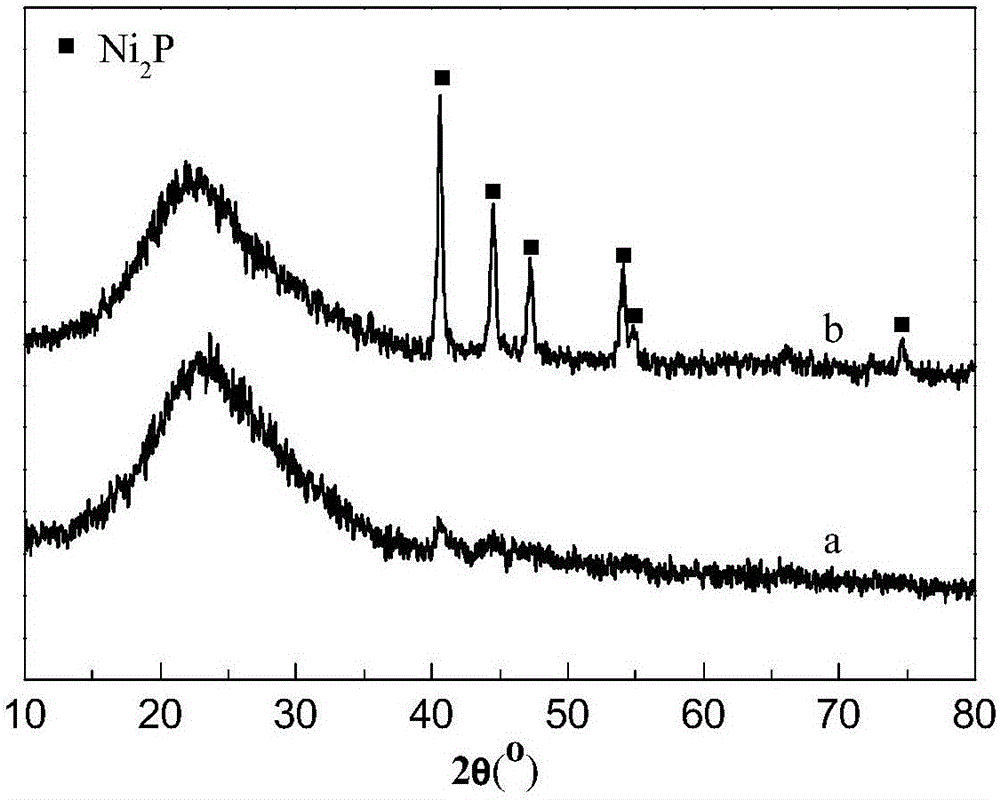
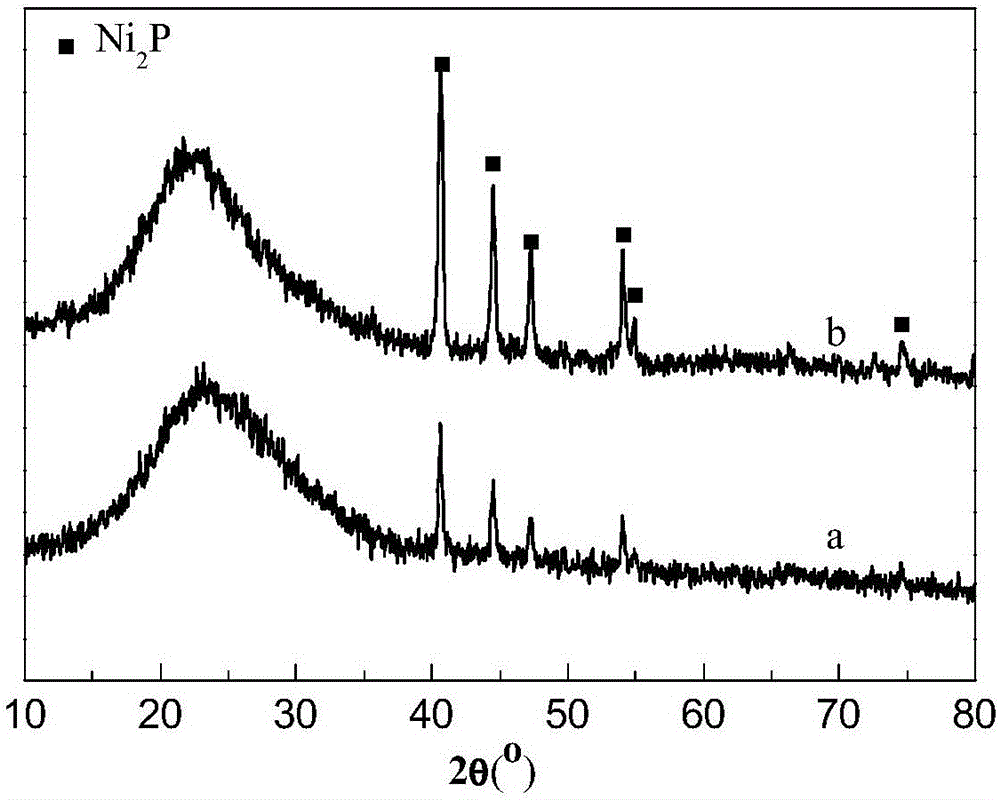
[0026] Embodiment 1 uses ammonium hypophosphite (NH 4 h 2 PO 2 ) as phosphorus source to prepare Ni 2 P / MCM-41 catalyst, and characterize the prepared catalyst. Specifically include the following steps:
[0027] (1)Ni 2 Preparation of P / MCM-41 catalyst precursor:
[0028] Dissolve nickel acetate (analytical pure, 99%) and ammonium hypophosphite (analytical pure, 99%) in distilled water according to the Ni / P molar ratio of 1:2, stir to obtain a suspension, and then add concentrated nitric acid to it to obtain clarification solution, then add MCM-41 mesoporous molecular sieves, impregnate for 12 hours, dry at 60°C for 30min, and then bake at 480°C for 3 hours to obtain Ni 2 P / MCM-41 catalyst precursor.
[0029] (2)Ni 2 Preparation of P / MCM-41 Catalyst
[0030] Ni prepared by step (1) 2 The P / MCM-41 catalyst precursor is placed in a fixed bed reactor, heated to 350°C in a hydrogen flow of 100mL / min at a rate of 2.5°C / min per minute, kept at this temperature for 2 hours,...
Embodiment 2
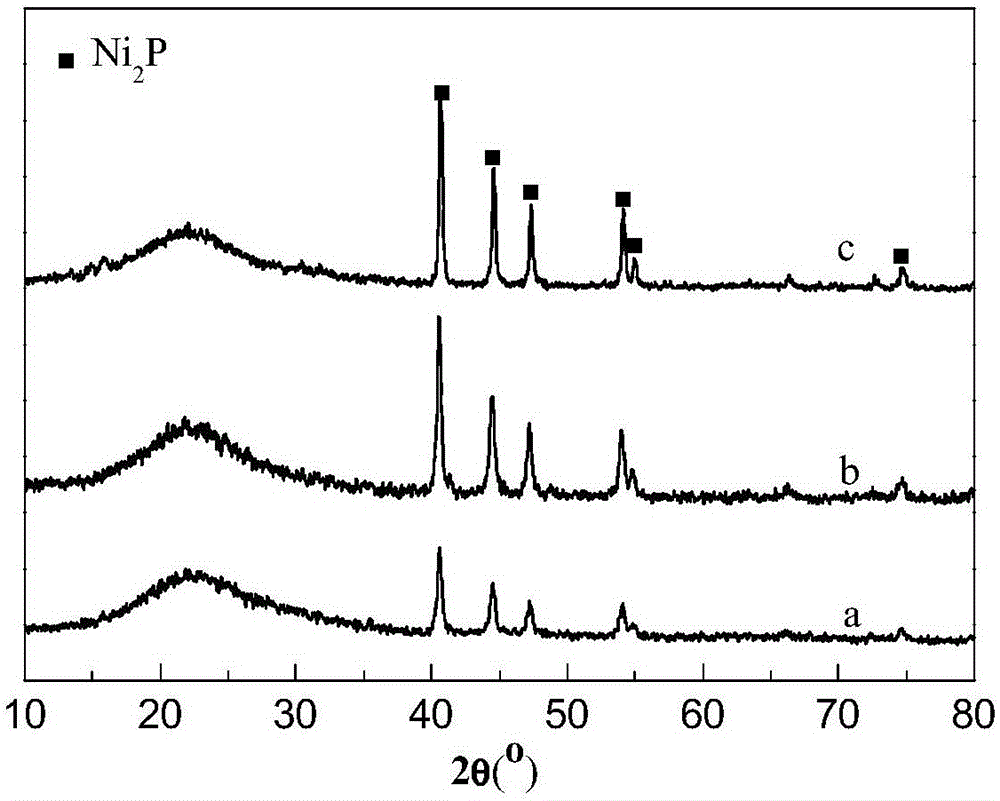
[0035] Example 2 provides Ni 2 The preparation method of P / MCM-41 catalyst, and the prepared catalyst is characterized. Specifically include the following steps:
[0036] (1)Ni 2 Preparation of P / MCM-41 Catalyst Precursor
[0037] Dissolve nickel acetate (analytical pure, 99%) and ammonium hypophosphite (analytical pure, 99%) in distilled water according to the Ni / P molar ratio of 1:4, stir to obtain a suspension, and then add concentrated nitric acid to it to obtain clarification solution, then add MCM-41 mesoporous molecular sieves, impregnate for 15 hours, dry at 40°C for 50min, and then bake at 500°C for 2 hours to obtain Ni 2 P / MCM-41 catalyst precursor.
[0038] (2)Ni 2 Preparation of P / MCM-41 Catalyst
[0039] Ni prepared by step (1) 2 The P / MCM-41 catalyst precursor was placed in a fixed bed reactor, heated to 350°C in a hydrogen flow of 120mL / min at a rate of 3°C / min per minute, kept at this temperature for 2.5 hours and then heated to 20°C / min rate to cool d...
Embodiment 3
[0041] The difference between embodiment 3 and embodiment 2 is only in Ni 2 During the preparation of the P / MCM-41 catalyst, the temperature in the fixed-bed reactor was heated to 400°C, and the rest of the steps and parameters were the same.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com