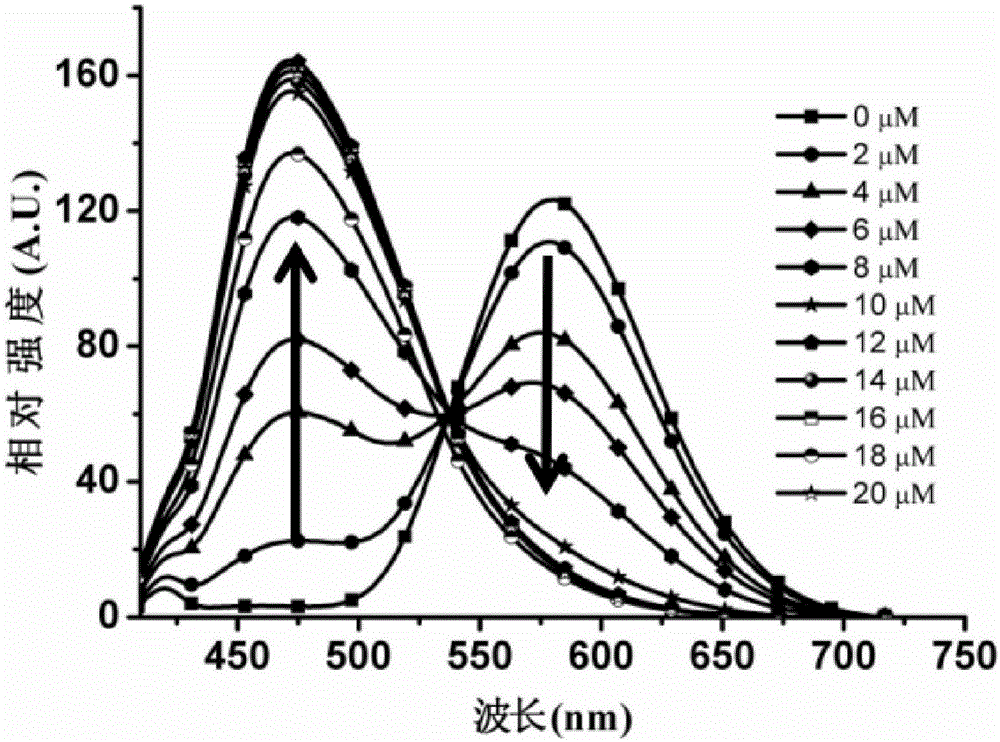
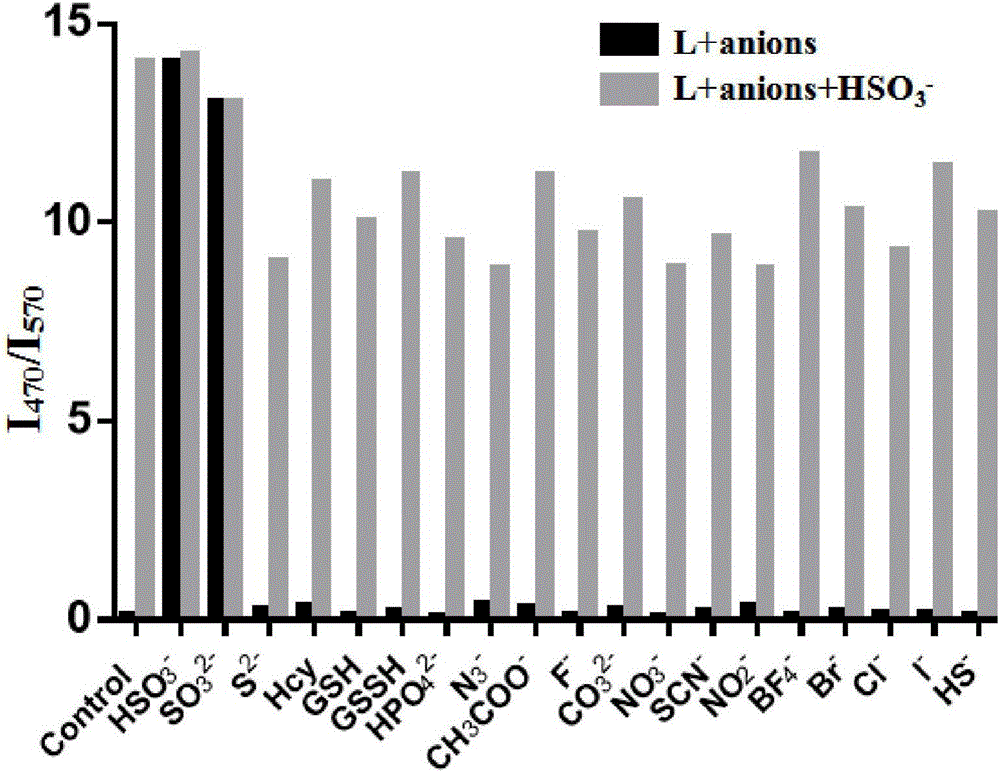
Two-photon fluorescent probe of benzoindole derivative and preparation method and application thereof
A benzoindole derivative, two-photon fluorescence technology, applied in fluorescence/phosphorescence, chemical instruments and methods, luminescent materials, etc., can solve the problems of inability to detect low concentration, complex sample pretreatment, low detection sensitivity, etc. Small sampling volume, high sensitivity, high sensitivity effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment Construction
[0040] The preparation method of the benzindole derivative two-photon fluorescent probe in this embodiment is as follows:
[0041] 1. Synthesis of intermediate OT
[0042] Add 3.51g (10mmol) of 2,3,3-trimethylbenzindole to a 50mL round-bottomed flask, add 2.8g (20mmol) of methyl iodide at room temperature, mix well and stir at room temperature for 24h. After the reaction A white precipitate is formed (if the precipitate contains a small amount of impurities, it can be washed with an appropriate amount of ethanol), and 3.3 g of a white solid is obtained by suction filtration, which is the intermediate OT, and the yield is 94%.
[0043] 2. Synthesis of target product L
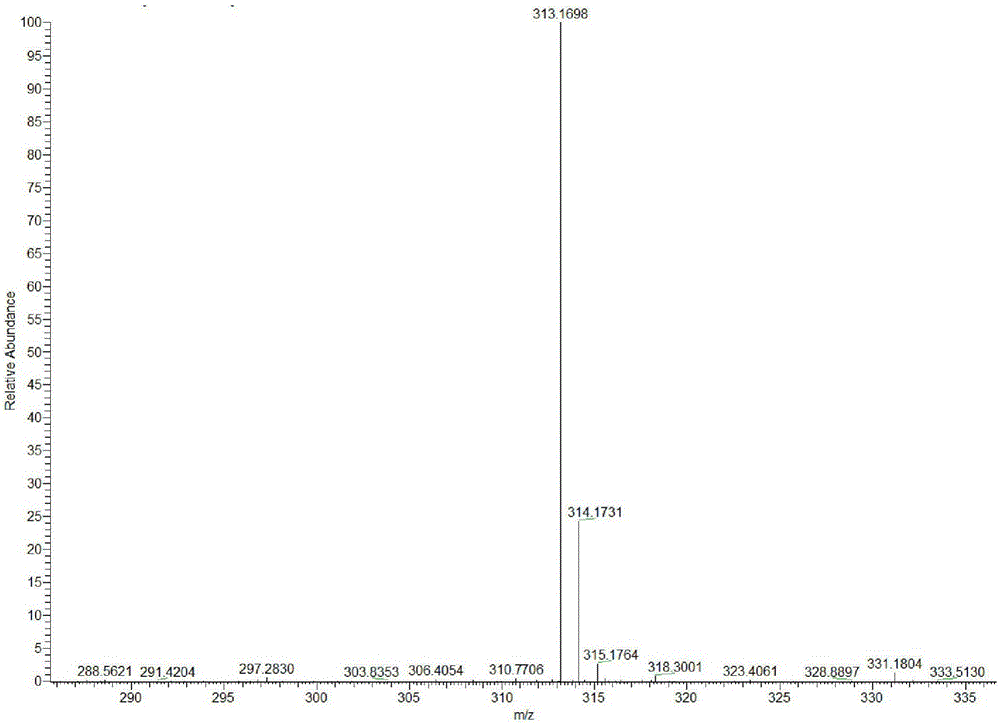
[0044] Add 3.51g (10mmol) of intermediate OT, 1.1g (10mmol) of 4-pyridinecarbaldehyde and 30mL of ethanol to a 100mL three-necked flask in turn, add 2 drops of catalyst hexahydropyridine under stirring, and then raise the temperature to 60°C for 6 hours. The solution turned purple, and a red solid was precipit...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com