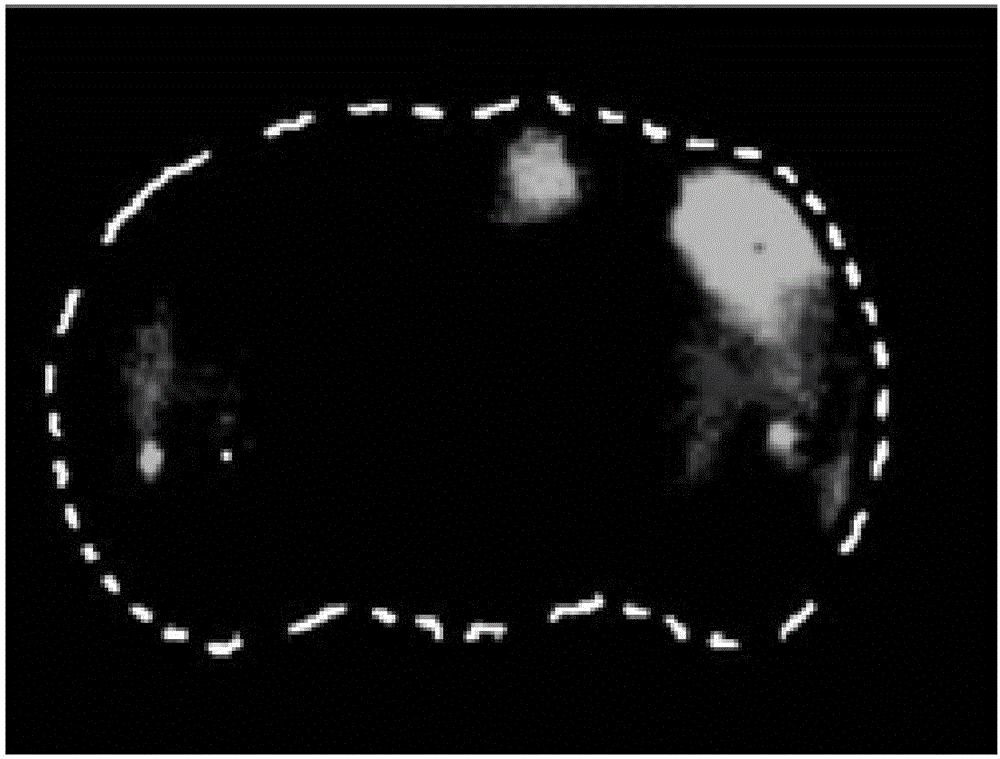
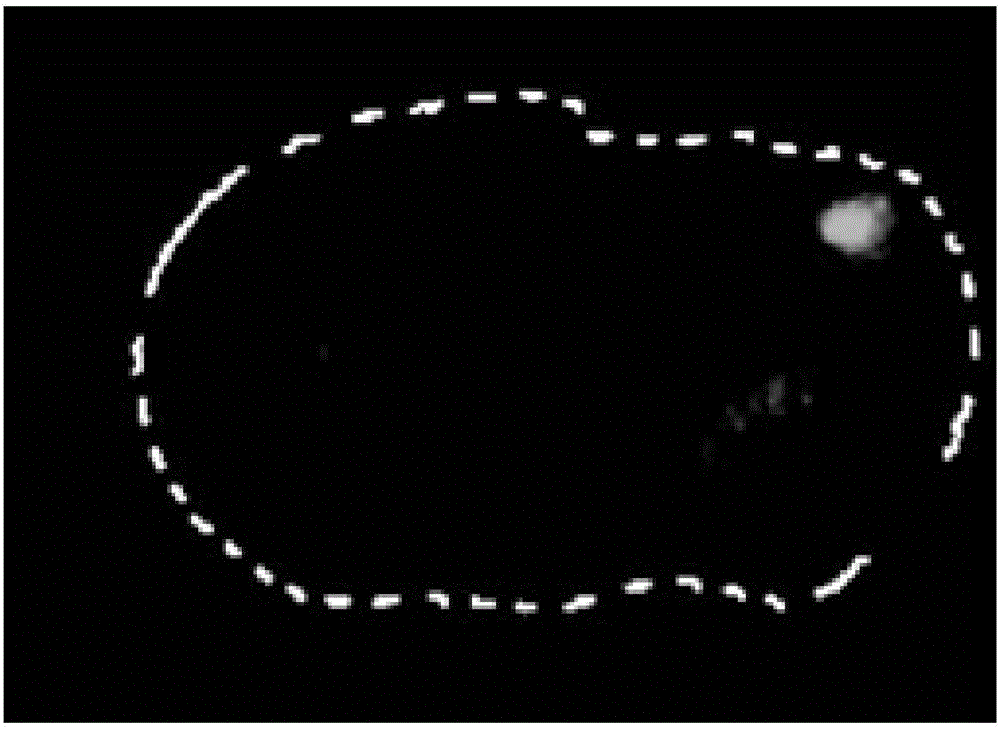
Polypeptide molecule with brain targeting function as well as preparation method and application thereof
A brain-targeting and molecular technology, applied in the field of polypeptide molecules with brain-targeting function and their preparation, can solve the problem that macromolecules cannot cross the blood-brain barrier, avoid adverse immune reactions in the human body, and improve the cure rate and speed. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0059] Liquid Phase Synthesis of CGQK (SEQ ID NO 5)
[0060] Selection of reagents used for synthesis:
[0061] (1) Amino acids: H-Cys(Trt)-OH, Fmoc-Gly-OH, Fmoc-Gln-OH, H-Lys(Boc)-Ome;
[0062] (2) Condensing agent: DIC or HOBT;
[0063] (3) Solvent: DCM;
[0064] (4) Condensation base: DMAP;
[0065] (5) Deprotection reagent: piperidine;
[0066] (6) Cutting fluid: TFA / TIS / H 2 O, the ratio is: 95:2.5:2.5;
[0067] The reaction is carried out in a round bottom flask, stirred with a magnet, the first step reaction:
[0068] Add H-Lys(Boc)-Ome, Fmoc-Gln-OH, condensing agent, and condensing base into the flask at the same time according to the ratio of 1:1:1:1, stir, control the temperature and react at 25°C for 4 hours, add Protecting agent to a concentration of 20%, react for 30 minutes, remove the reaction solution by rotary evaporation, add the solvent DCM for the next reaction: add Fmoc-Gly-OH, condensing agent, and condensing base to the flask according to the ratio...
Embodiment 2
[0070] Solid phase synthesis of CAQKCAQKCAQKCAQKCAQK (SEQ ID NO 4)
[0071] CAQKCAQKCAQKCAQKCAQK (SEQ ID NO 4) was synthesized using the solid-phase peptide synthesis method of the Fmoc strategy, using a Biotage instrument produced by SyroII Company. The operation method was carried out according to the manufacturer's instruction manual.
[0072] Selection of reagents used for synthesis:
[0073] (1) Solid phase resin: 2-cl-trt resin, resin substitution degree 0.75;
[0074] (2) Protected amino acids: Fmoc-Cys(Boc)-OH, Fmoc-Ala-OH, Fmoc-Gln-OH, Fmoc-Lys(Boc)-OH;
[0075] (3) Deprotection reagent: piperidine, the concentration of piperidine in the solution used is 15%-50%; in a preferred embodiment, the concentration of piperidine in the solution used is 20%;
[0076] (4) Coupling reagent: HATU / HOBT / DIEA, the ratio is 1:1:2
[0077] (5) Cutting reagent: TFA / TIS / H 2 O, the ratio is: 95:2.5:2.5;
[0078] The crude peptide was purified by HPLC. Purification conditions: grad...
Embodiment 3
[0080] Solid phase synthesis of CAQKCAQK (SEQ ID NO 2)
[0081] CAQKCAQK (SEQ ID NO 2) was synthesized using the solid-phase peptide synthesis method of the Fmoc strategy, using a Biotage instrument produced by SyroII Company. The operation method was carried out according to the manufacturer's instruction manual.
[0082] Selection of reagents used for synthesis:
[0083] (1) Carrier resin: Fmoc-Lys-wang-rink, degree of substitution: 0.8
[0084] (2) Protected amino acids: Fmoc-Cys(Boc)-OH, Fmoc-Ala-OH, Fmoc-Gln-OH, Fmoc-Lys(Boc)-OH, 5-fold excess of protected amino acids used in the reaction.
[0085] (3) Deprotection reagent: piperidine / N,N-dimethylformamide, the ratio is 20:80.
[0086] (4) Coupling reagent: DIC / HOBT, the ratio is 1:1.5.
[0087] (5) Cutting reagent: TFA / TIS / water, the ratio is: 95:2.5:2.5.
[0088] The prepared polypeptide was purified using a HPLCC18 semi-preparative column, and the detection conditions were: detection wavelength: 214nm, mobile phas...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com