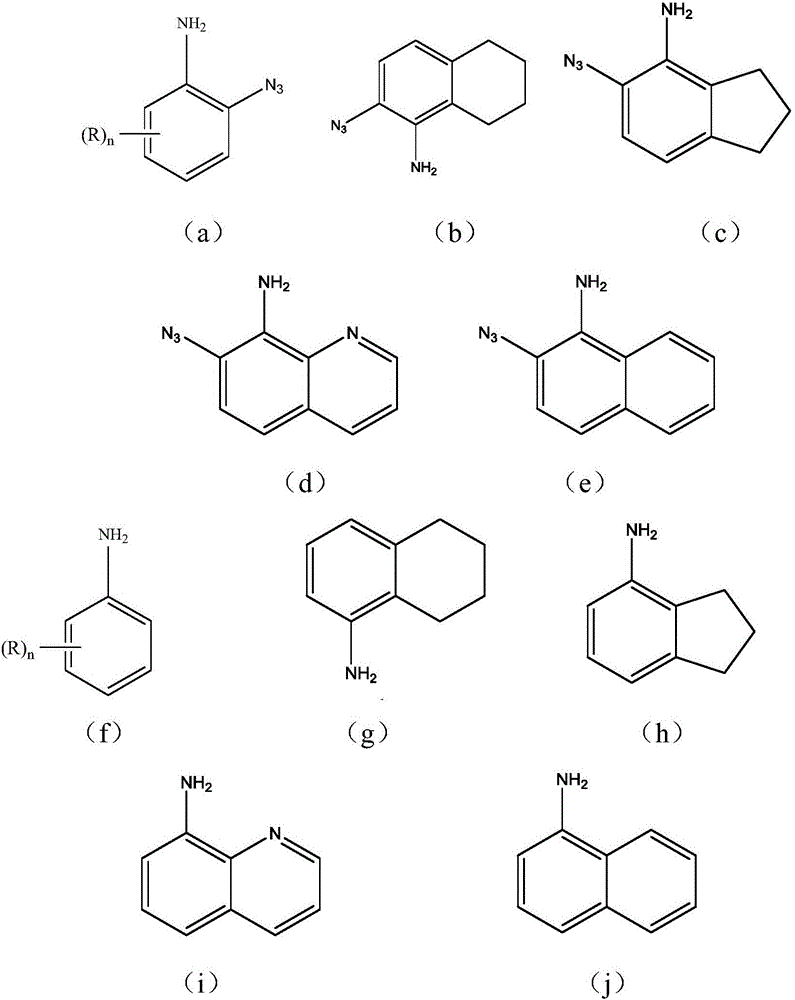
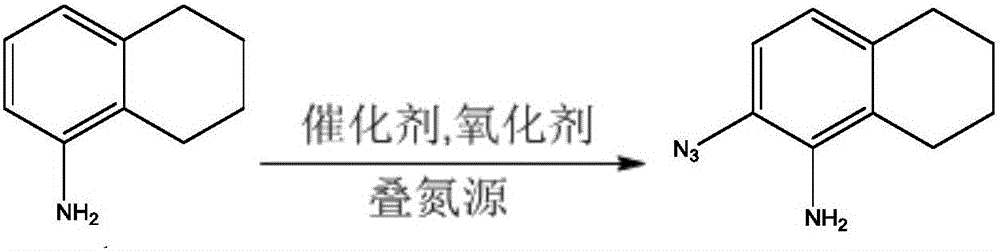
Method for preparing azide compound through azidation based on C-H activated anilines
A compound and nitrogen source technology, applied in organic chemistry methods, chemical instruments and methods, organic chemistry, etc., can solve problems such as harsh conditions, reduce the progress of azide compounds, environmental pollution, etc., and achieve the effect of simple operation process
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0034] Preparation of 2-azidoaniline (2-azidoaniline)
[0035]
[0036] Add 0.5mmol of aniline (1a) into 4mL of water, add 0.125mmol of copper acetate, 1.0mmol of sodium azide, and 1.0mmol of hydrogen peroxide, and react at room temperature for 2 hours. After the reaction, add saturated NaCl aqueous solution to the reaction solution , extracted with ethyl acetate, the organic layer was dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness at 60° C. under reduced pressure to obtain the crude compound represented by the formula (2a). The crude compound represented by formula (2a) was subjected to silica gel column chromatography, and a solution with a volume ratio of ethyl acetate and petroleum ether of 1:5 was used as the mobile phase, and the eluent with an Rf value of 0.3-0.5 was tracked and collected by TLC. The obtained eluate was desolventized under reduced pressure and dried to obtain 42 mg of the pure compound represented by formula (2a), with a ...
Embodiment 2
[0039] Preparation of 2-azido-3-bromoaniline (2-azido-3-bromoaniline)
[0040]
[0041] Add 0.5mmol m-bromoaniline (1b) into 4mL of water, add 0.125mmol copper acetate, 1.0mmol sodium azide, 1.0mmol hydrogen peroxide, and react at room temperature for 2 hours. After the reaction, add saturated NaCl aqueous solution was extracted with ethyl acetate, the organic layer was dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness at 60°C under reduced pressure to obtain the crude compound represented by the formula (2b). The crude compound represented by formula (2b) was subjected to silica gel column chromatography, and the solution with a volume ratio of ethyl acetate and petroleum ether of 1:8 was used as the mobile phase, and the eluent with an Rf value of 0.3-0.5 was tracked and collected by TLC. The obtained eluent was desolventized under reduced pressure and dried to obtain 69 mg of the pure compound represented by formula (2b), with a yield of 65%.
...
Embodiment 3
[0044] Preparation of 2-azido-3-chloroaniline (2-azido-3-chloroaniline)
[0045]
[0046] Add 0.5mmol m-chloroaniline (1c) to 4mL of water, add 0.125mmol copper acetate, 1.0mmol sodium azide, 1.0mmol hydrogen peroxide, and react at room temperature for 2 hours. After the reaction, add saturated NaCl aqueous solution was extracted with ethyl acetate, the organic layer was dried over anhydrous magnesium sulfate, filtered, and evaporated to dryness at 60°C under reduced pressure to obtain the crude compound represented by the formula (2c). The crude compound represented by formula (2c) was subjected to silica gel column chromatography, using a solution with a volume ratio of ethyl acetate and petroleum ether of 1:6 as the mobile phase, and followed by TLC to collect the eluent with an Rf value of 0.3-0.5, and collected The obtained eluent was desolventized under reduced pressure and dried to obtain 55 mg of the pure compound represented by formula (2c), with a yield of 66%.
...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com